Long-term outcomes of flow diversion for unruptured intracranial aneurysms: a systematic review and meta-analysis
- PMID: 36150896
- PMCID: PMC10033458
- DOI: 10.1136/jnis-2022-019240
Long-term outcomes of flow diversion for unruptured intracranial aneurysms: a systematic review and meta-analysis
Abstract
Background: Flow diverters have been widely used in clinical practice for more than a decade. However, most outcome data are limited to 1 year timepoints. This study aims to offer meta-analysis data on long-term (>1 year) safety and effectiveness results for patients with aneurysms treated with flow diverters.
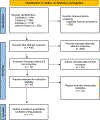
Methods: PubMed, Web of Science, Embase, and SCOPUS were searched up to February 24, 2022 using the AutoLit platform. We included primary studies assessing the long-term outcomes for flow diverter devices to manage unruptured internal carotid artery aneurysms with a follow-up period of >1 year. The meta-analysis was carried out using Comprehensive Meta-Analysis software (CMA).
Results: Eleven studies were included in the meta-analysis. The pooled occlusion rates after flow diversion treatment for unruptured intracranial brain aneurysms were 77%, 87.4%, 84.5%, 89.4%, 96% for 1 year, 1-2 years, 2 years, 3 years, and 5 years follow-up, respectively. The in-stent stenosis rate was 4.8% and the retreatment rate for the long-term follow-up period was 5%. No delayed rupture of the aneurysm was reported, and there was one case of delayed ischemic stroke. The sensitivity analysis of the prospective studies showed a complete occlusion rate of 83.5% and 85.2% for 1 and 3 years of follow-up, respectively.
Conclusion: Flow diverters are safe and effective in short- and long-term follow-up and rarely cause serious delayed side effects.
Keywords: aneurysm.
© Author(s) (or their employer(s)) 2023. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DFK holds equity in Nested Knowledge, Superior Medical Editors, and Conway Medical, Marblehead Medical; is a consultant for MicroVention, Medtronic, Balt, and Insera Therapeutics; Data Safety Monitoring Board for Vesalio; and receives royalties from Medtronic. RK reports NIH funding (R01 NS076491, R43 NS110114, and R44 NS107111), is a research consultant for Cerenovus, Insera Therapeutics, Marblehead Medical, Microvention, MIVI Neuroscience, Neurogami Medical, and Triticum, and has stock in Neurosigma (money paid to institution).
Figures
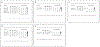
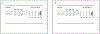
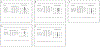
References
-
- Kühn AL, Gounis MJ, Puri AS. Introduction: History and Development of Flow Diverter Technology and Evolution. Neurosurgery. 2020. Jan 1;86(Suppl 1):S3–10. - PubMed
-
- Zhou G, Su M, Yin YL, Li MH. Complications associated with the use of flow-diverting devices for cerebral aneurysms: a systematic review and meta-analysis. Neurosurg Focus. 2017. Jun;42(6):E17. - PubMed
-
- Kotowski M, Naggara O, Darsaut TE, Nolet S, Gevry G, Kouznetsov E, et al. Safety and occlusion rates of surgical treatment of unruptured intracranial aneurysms: a systematic review and meta-analysis of the literature from 1990 to 2011. J Neurol Neurosurg Psychiatry. 2013. Jan;84(1):42–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
