Pathogenesis of Zika Virus Infection
- PMID: 36151059
- PMCID: PMC10893765
- DOI: 10.1146/annurev-pathmechdis-031521-034739
Pathogenesis of Zika Virus Infection
Abstract
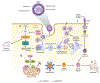
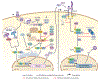
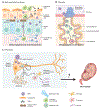
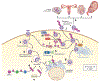
Zika virus (ZIKV) is an emerging virus from the Flaviviridae family that is transmitted to humans by mosquito vectors and represents an important health problem. Infections in pregnant women are of major concern because of potential devastating consequences during pregnancy and have been associated with microcephaly in newborns. ZIKV has a unique ability to use the host machinery to promote viral replication in a tissue-specific manner, resulting in characteristic pathological disorders. Recent studies have proposed that the host ubiquitin system acts as a major determinant of ZIKV tropism by providing the virus with an enhanced ability to enter new cells. In addition, ZIKV has developed mechanisms to evade the host immune response, thereby allowing the establishment of viral persistence and enhancing viral pathogenesis. We discuss recent reports on the mechanisms used by ZIKV to replicate efficiently, and we highlight potential new areas of research for the development of therapeutic approaches.
Keywords: TRIM7; Zika virus; antagonism of immune responses; pathogenesis; tropism; ubiquitin.
Figures
Similar articles
-
Zika virus cell tropism in the developing human brain and inhibition by azithromycin.Proc Natl Acad Sci U S A. 2016 Dec 13;113(50):14408-14413. doi: 10.1073/pnas.1618029113. Epub 2016 Nov 29. Proc Natl Acad Sci U S A. 2016. PMID: 27911847 Free PMC article.
-
Congenital Abnormalities: Consequence of Maternal Zika Virus Infection: A Narrative Review.Infect Disord Drug Targets. 2017;17(1):3-13. doi: 10.2174/1871526516666161018104916. Infect Disord Drug Targets. 2017. PMID: 27758685 Review.
-
Zika virus and microcephaly in Southeast Asia: A cause for concern?J Infect Public Health. 2020 Jan;13(1):11-15. doi: 10.1016/j.jiph.2019.09.012. Epub 2019 Oct 24. J Infect Public Health. 2020. PMID: 31669035 Review.
-
Perinatal analyses of Zika- and dengue virus-specific neutralizing antibodies: A microcephaly case-control study in an area of high dengue endemicity in Brazil.PLoS Negl Trop Dis. 2019 Mar 11;13(3):e0007246. doi: 10.1371/journal.pntd.0007246. eCollection 2019 Mar. PLoS Negl Trop Dis. 2019. PMID: 30856223 Free PMC article.
-
Persistent detection of Zika virus RNA from an infant with severe microcephaly - a case report.BMC Infect Dis. 2018 Aug 10;18(1):388. doi: 10.1186/s12879-018-3313-4. BMC Infect Dis. 2018. PMID: 30097025 Free PMC article.
Cited by
-
Zika virus transmission in Aedes aegypti: A systematic study on the ability of mosquitoes to transmit the virus horizontally and vertically.Virol Sin. 2025 Apr;40(2):192-205. doi: 10.1016/j.virs.2025.02.001. Epub 2025 Feb 11. Virol Sin. 2025. PMID: 39947399 Free PMC article.
-
Common arboviruses and the kidney: a review.J Bras Nefrol. 2024 Jul-Sep;46(3):e20230168. doi: 10.1590/2175-8239-JBN-2023-0168en. J Bras Nefrol. 2024. PMID: 39074252 Free PMC article. Review.
-
A review on Zika vaccine development.Pathog Dis. 2024 Feb 7;82:ftad036. doi: 10.1093/femspd/ftad036. Pathog Dis. 2024. PMID: 38192053 Free PMC article. Review.
-
Inactivation of Zika Virus with Hydroxypropyl-Beta-Cyclodextrin.Vaccines (Basel). 2025 Jan 16;13(1):79. doi: 10.3390/vaccines13010079. Vaccines (Basel). 2025. PMID: 39852858 Free PMC article.
-
Intrauterine Zika Virus Infection: An Overview of the Current Findings.J Pers Med. 2025 Mar 1;15(3):98. doi: 10.3390/jpm15030098. J Pers Med. 2025. PMID: 40137414 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

