Genetically modified CD7-targeting allogeneic CAR-T cell therapy with enhanced efficacy for relapsed/refractory CD7-positive hematological malignancies: a phase I clinical study
- PMID: 36151216
- PMCID: PMC9652391
- DOI: 10.1038/s41422-022-00721-y
Genetically modified CD7-targeting allogeneic CAR-T cell therapy with enhanced efficacy for relapsed/refractory CD7-positive hematological malignancies: a phase I clinical study
Abstract
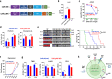
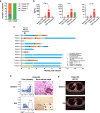
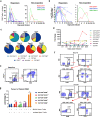
Chimeric antigen receptor (CAR)-T cell therapy against T cell malignancies faces major challenges including fratricide between CAR-T cells and product contamination from the blasts. Allogeneic CAR-T cells, generated from healthy donor T cells, can provide ready-to-use, blast-free therapeutic products, but their application could be complicated by graft-versus-host disease (GvHD) and host rejection. Here we developed healthy donor-derived, CD7-targeting CAR-T cells (RD13-01) with genetic modifications to resist fratricide, GvHD and allogeneic rejection, as well as to potentiate antitumor function. A phase I clinical trial (NCT04538599) was conducted with twelve patients recruited (eleven with T cell leukemia/lymphoma, and one with CD7-expressing acute myeloid leukemia). All patients achieved pre-set end points and eleven proceeded to efficacy evaluation. No dose-limiting toxicity, GvHD, immune effector cell-associated neurotoxicity or severe cytokine release syndrome (grade ≥ 3) were observed. 28 days post infusion, 81.8% of patients (9/11) showed objective responses and the complete response rate was 63.6% (7/11, including the patient with AML). 3 of the responding patients were bridged to allogeneic hematopoietic stem cell transplantation. With a median follow-up of 10.5 months, 4 patients remained in complete remission. Cytomegalovirus (CMV) and/or Epstein-Barr virus (EBV) reactivation was observed in several patients, and one died from EBV-associated diffuse large B-cell lymphoma (DLBCL). Expansion of CD7-negative normal T cells was detected post infusion. In summary, we present the first report of a Phase I clinical trial using healthy donor-derived CD7-targeting allogeneic CAR-T cells to treat CD7+ hematological malignancies. Our results demonstrated the encouraging safety and efficacy profiles of the RD13-01 allogeneic CAR-T cells for CD7+ tumors.
© 2022. The Author(s) under exclusive licence to Center for Excellence in Molecular Cell Science, CAS.
Conflict of interest statement
Y.Z., W.G., G.C., L.H., T.G., X.J., X.Zheng, S.Y., Xiaolong L., X.Zhang, M.C., Xiuju L., M.G., K.W., X.H., Y.W., and J.R. are employees of Nanjing Bioheng Biotech Co. All other authors declare no competing interests.
Figures


References
-
- Rodriguez-Zuniga MJM, Cortez-Franco F, Qujiano-Gomero E. Adult T-cell leukemia/lymphoma. Review of the literature. Actas Dermosifiliogr (Engl Ed) 2018;109:399–407. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

