Novel D-form of hybrid peptide (D-AP19) rapidly kills Acinetobacter baumannii while tolerating proteolytic enzymes
- PMID: 36151303
- PMCID: PMC9508196
- DOI: 10.1038/s41598-022-20236-1
Novel D-form of hybrid peptide (D-AP19) rapidly kills Acinetobacter baumannii while tolerating proteolytic enzymes
Erratum in
-
Author Correction: Novel D-form of hybrid peptide (D-AP19) rapidly kills Acinetobacter baumannii while tolerating proteolytic enzymes.Sci Rep. 2023 Feb 23;13(1):3154. doi: 10.1038/s41598-023-30354-z. Sci Rep. 2023. PMID: 36823306 Free PMC article. No abstract available.
Abstract
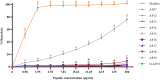
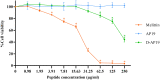
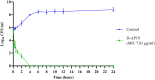
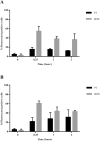
Antimicrobial peptides (AMPs) are being developed as potent alternative treatments to conventional antibiotics which are unlikely to induce bacterial resistance. They can be designed and modified to possess several druggable properties. We report herein a novel hybrid peptide of modified aurein (A3) and cathelicidin (P7), or A3P7, by a flipping technique. It exhibited potent antibacterial activity against both Gram-negative and -positive pathogenic bacteria but had moderate hemolytic activity. To reduce the sequence length and toxicity, C-terminal truncation was serially performed and eight truncated derivatives (AP12-AP19) were obtained. They had significantly less hemolytic activity while preserving antibacterial activity. Secondary structures of the candidate peptides in environments simulating bacterial membranes (30 mM SDS and 50% TFE), determined by CD spectroscopy, showed α-helical structures consistent with predicted in silico 3D structural models. Among the peptides, AP19 demonstrated the best combination of broad-spectrum antibacterial activity (including toward Acinetobacter baumannii) and minimal hemolytic and cytotoxic activities. A D-form peptide (D-AP19), in which all L-enantiomers were substituted with the D-enantiomers, maintained antibacterial activity in the presence of pepsin, trypsin, proteinase K and human plasma. Both isomers exhibited potent antibacterial activity against multi-drug (MDR) and extensively-drug resistant (XDR) clinical isolates of A. baumannii comparable to the traditional antibiotic, meropenem. D-AP19 displayed rapid killing via membrane disruption and leakage of intracellular contents. Additionally, it showed a low tendency to induce bacterial resistance. Our work suggested that D-AP19 could be further optimized and developed as a novel compound potentially for fighting against MDR or XDR A. baumannii.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Deciphering the Intracellular Action of the Antimicrobial Peptide A11 via an In-Depth Analysis of Its Effect on the Global Proteome of Acinetobacter baumannii.ACS Infect Dis. 2024 Aug 9;10(8):2795-2813. doi: 10.1021/acsinfecdis.4c00160. Epub 2024 Jul 29. ACS Infect Dis. 2024. PMID: 39075773 Free PMC article.
-
Antimicrobial and Anti-Inflammatory Activities of MAF-1-Derived Antimicrobial Peptide Mt6 and Its D-Enantiomer D-Mt6 against Acinetobacter baumannii by Targeting Cell Membranes and Lipopolysaccharide Interaction.Microbiol Spectr. 2022 Oct 26;10(5):e0131222. doi: 10.1128/spectrum.01312-22. Epub 2022 Oct 3. Microbiol Spectr. 2022. PMID: 36190276 Free PMC article.
-
Peptides from American alligator plasma are antimicrobial against multi-drug resistant bacterial pathogens including Acinetobacter baumannii.BMC Microbiol. 2016 Aug 19;16(1):189. doi: 10.1186/s12866-016-0799-z. BMC Microbiol. 2016. PMID: 27542832 Free PMC article.
-
Antimicrobial peptides as a promising treatment option against Acinetobacter baumannii infections.Microb Pathog. 2020 Sep;146:104238. doi: 10.1016/j.micpath.2020.104238. Epub 2020 May 5. Microb Pathog. 2020. PMID: 32387392 Review.
-
Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli.Drugs. 2019 Feb;79(3):271-289. doi: 10.1007/s40265-019-1055-2. Drugs. 2019. PMID: 30712199 Review.
Cited by
-
Deciphering the Intracellular Action of the Antimicrobial Peptide A11 via an In-Depth Analysis of Its Effect on the Global Proteome of Acinetobacter baumannii.ACS Infect Dis. 2024 Aug 9;10(8):2795-2813. doi: 10.1021/acsinfecdis.4c00160. Epub 2024 Jul 29. ACS Infect Dis. 2024. PMID: 39075773 Free PMC article.
-
Protease-Resistant, Broad-Spectrum Antimicrobial Peptides with High Antibacterial and Antifungal Activity.Life (Basel). 2025 Feb 5;15(2):242. doi: 10.3390/life15020242. Life (Basel). 2025. PMID: 40003651 Free PMC article.
-
Hybrids of Membrane-Translocating Antimicrobial Peptides Show Enhanced Activity through Membrane Permeabilization.ACS Med Chem Lett. 2024 Oct 11;15(11):1918-1924. doi: 10.1021/acsmedchemlett.4c00375. eCollection 2024 Nov 14. ACS Med Chem Lett. 2024. PMID: 39563817 Free PMC article.
-
An Update on the Therapeutic Potential of Antimicrobial Peptides against Acinetobacter baumannii Infections.Pharmaceuticals (Basel). 2023 Sep 11;16(9):1281. doi: 10.3390/ph16091281. Pharmaceuticals (Basel). 2023. PMID: 37765087 Free PMC article. Review.
-
Promising applications of D-amino acids in periprosthetic joint infection.Bone Res. 2023 Mar 10;11(1):14. doi: 10.1038/s41413-023-00254-z. Bone Res. 2023. PMID: 36894568 Free PMC article. Review.
References
-
- Khan HA, Baig FK, Mehboob R. Nosocomial infections: epidemiology, prevention, control and surveillance. Asian. Pac. J. Trop. Biomed. 2017;7:478–482. doi: 10.1016/j.apjtb.2017.01.019. - DOI
-
- World Health Organization. WHO publishes list of bacteria for which new antibiotics are urgently needed. (2017) Available at, https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-... (Date of access: 13/05/2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

