WNK1/HSN2 mediates neurite outgrowth and differentiation via a OSR1/GSK3β-LHX8 pathway
- PMID: 36151370
- PMCID: PMC9508073
- DOI: 10.1038/s41598-022-20271-y
WNK1/HSN2 mediates neurite outgrowth and differentiation via a OSR1/GSK3β-LHX8 pathway
Abstract
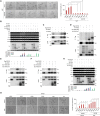
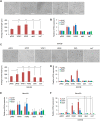
With no lysine kinase 1 (WNK1) phosphorylates and activates STE20/SPS1-related proline-alanine-rich protein kinase (SPAK) and oxidative stress responsive kinase 1 (OSR1) to regulate ion homeostasis in the kidney. Mutations in WNK1 result in dysregulation of the WNK1-SPAK/OSR1 pathway and cause pseudohypoaldosteronism type II (PHAII), a form of hypertension. WNK1 is also involved in the autosomal recessive neuropathy, hereditary sensory and autonomic neuropathy type II (HSANII). Mutations in a neural-specific splice variant of WNK1 (HSN2) cause HSANII. However, the mechanisms underlying HSN2 regulation in neurons and effects of HSN2 mutants remain unclear. Here, we found that HSN2 regulated neurite outgrowth through OSR1 activation and glycogen synthase kinase 3β (GSK3β). Moreover, HSN2-OSR1 and HSN2-GSK3β signalling induced expression of LIM homeobox 8 (Lhx8), which is a key regulator of cholinergic neural function. The HSN2-OSR1/GSK3β-LHX8 pathway is therefore important for neurite outgrowth. Consistently, HSN2 mutants reported in HSANII patients suppressed SPAK and OSR1 activation and LHX8 induction. Interestingly, HSN2 mutants also suppressed neurite outgrowth by preventing interaction of between wild-type HSN2 and GSK3β. These results indicate that HSN2 mutants cause dysregulation of neurite outgrowth via GSK3β in the HSN2 and/or WNK1 pathways.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
WNK1/HSN2 mutation in human peripheral neuropathy deregulates KCC2 expression and posterior lateral line development in zebrafish (Danio rerio).PLoS Genet. 2013;9(1):e1003124. doi: 10.1371/journal.pgen.1003124. Epub 2013 Jan 3. PLoS Genet. 2013. PMID: 23300475 Free PMC article.
-
The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases.Biochem J. 2005 Oct 1;391(Pt 1):17-24. doi: 10.1042/BJ20051180. Biochem J. 2005. PMID: 16083423 Free PMC article.
-
WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1.J Biol Chem. 2005 Dec 30;280(52):42685-93. doi: 10.1074/jbc.M510042200. Epub 2005 Oct 31. J Biol Chem. 2005. PMID: 16263722
-
Kelch-like 3/Cullin 3 ubiquitin ligase complex and WNK signaling in salt-sensitive hypertension and electrolyte disorder.Nephrol Dial Transplant. 2016 Sep;31(9):1417-24. doi: 10.1093/ndt/gfv259. Epub 2015 Jul 6. Nephrol Dial Transplant. 2016. PMID: 26152401 Review.
-
Regulation of with-no-lysine kinase signaling by Kelch-like proteins.Biol Cell. 2014 Feb;106(2):45-56. doi: 10.1111/boc.201300069. Epub 2014 Jan 10. Biol Cell. 2014. PMID: 24313290 Free PMC article. Review.
Cited by
-
RNA isoform expression landscape of the human dorsal root ganglion generated from long-read sequencing.Pain. 2024 Nov 1;165(11):2468-2481. doi: 10.1097/j.pain.0000000000003255. Epub 2024 May 16. Pain. 2024. PMID: 38809314
-
A novel mutation in the WNK1/HSN2 gene causing hereditary sensory and autonomic neuropathy type 2 in Chinese patient.J Hum Genet. 2025 Apr;70(4):223-226. doi: 10.1038/s10038-024-01310-0. Epub 2024 Dec 23. J Hum Genet. 2025. PMID: 39710744
-
RNA isoform expression landscape of the human dorsal root ganglion (DRG) generated from long read sequencing.bioRxiv [Preprint]. 2023 Nov 1:2023.10.28.564535. doi: 10.1101/2023.10.28.564535. bioRxiv. 2023. Update in: Pain. 2024 Nov 1;165(11):2468-2481. doi: 10.1097/j.pain.0000000000003255. PMID: 37961262 Free PMC article. Updated. Preprint.
-
Evidence for Functional Regulation of the KLHL3/WNK Pathway by O-GlcNAcylation.bioRxiv [Preprint]. 2025 Feb 27:2025.02.27.640596. doi: 10.1101/2025.02.27.640596. bioRxiv. 2025. PMID: 40060460 Free PMC article. Preprint.
References
-
- Xu BE, et al. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J. Biol. Chem. 2000;275:16795–16801. - PubMed
-
- Veríssimo F, Jordan P. WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene. 2001;20:5562–5569. - PubMed
-
- Wilson FH, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. - PubMed
-
- Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1) J. Biol. Chem. 2002;277:50812–50819. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

