Efficacy and safety of apremilast and phototherapy versus phototherapy only in psoriasis vulgaris
- PMID: 36151864
- PMCID: PMC10087908
- DOI: 10.1111/1346-8138.16566
Efficacy and safety of apremilast and phototherapy versus phototherapy only in psoriasis vulgaris
Abstract
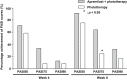
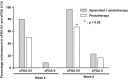
Phototherapy and apremilast (oral phosphodiesterase-4 inhibitor) are well-known in the treatment of moderate to severe psoriasis vulgaris. However, current evidence on the efficacy and safety of their combination is not sufficient. This multicenter, randomized controlled study compared the efficacy and safety between phototherapy as monotherapy and phototherapy and apremilast as combination therapy in patients with psoriasis vulgaris. Patients with moderate to severe psoriasis vulgaris were assigned to combination (n = 29) and monotherapy (n = 13) groups. All patients underwent an 8-week phototherapy regimen comprising irradiation with narrowband UV-B. The patients in the combination group were also administered 10 mg to 60 mg of oral apremilast. We evaluated the improvement percentage based on the Psoriasis Area and Severity Index (PASI) score from baseline to week 8. Additionally, we evaluated the percentage of patients who achieved ≥75% improvement; changes in body surface area (BSA) and scores of EuroQol 5-dimensions 5-level, Dermatology Life Quality Index, and visual analog scale for pruritis from baseline to 4 and 8 weeks; and adverse events. Compared with the monotherapy group, the combination group had significantly lower PASI scores at 4 and 8 weeks and more patients who achieved a PASI score improvement of ≥75% at 8 weeks. Both groups exhibited a significant decrease in BSA; at 8 weeks, no significant difference was observed between the two groups, although the combination group tended toward a greater reduction in BSA. The intergroup differences in the changes at the three time points were not significant. Adverse events were more frequent in the combination group than in the monotherapy group. Our findings suggest that an 8-week combined apremilast and phototherapy regimen may not be adequate in patients for improvements in their subjective assessment of psoriasis, and longer treatment periods may be necessary.
Keywords: Psoriasis Area and Severity Index; apremilast; phosphodiesterase-4 inhibitor; phototherapy; psoriasis.
© 2022 The Authors. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Conflict of interest statement
This research was funded by Amgen K.K. Tokyo, Japan. Akimichi Morita has received research grants, consulting fees, and/or speaker's fee from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Eisai, Janssen, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Nichi‐Iko, Nippon Kayaku, Novartis, Pfizer, Sun Pharmaceutical Industries Taiho Pharmaceutical, Torii Pharmaceutical, Ushio and UCB Pharma. Yukie Yamaguchi declares receiving research grants, and/or consulting fees, and/or speaker's fees from AbbVie, Amgen, Astellas, Boehringer Ingelheim, Eisai, Eli Lilly, Janssen, Kyowa Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Novartis, Sun Pharmaceutical Industries, Taiho Pharmaceutical, Torii Pharmaceutical, and UCB Japan. Chiharu Tateishi has received research grant, consulting fee, and/or speaker's fee from Amgen. Daisuke Hayashi has received research grant, consulting fee from Amgen. Yuko Watanabe has received speaker's fee from AbbVie, Eli Lilly, Maruho, Novartis, Taiho Pharmaceutical and UCB Pharma. Koji Masuda has received research grants from Eli Lilly Japan. Daisuke Tsuruta has received research grants and/or speaker's fee from Abbvie, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Eisai, Janssen, JIMRO Co., Ltd., Kyowa Hakko Kirin, Maruho, Mitsubishi Tanabe, Nippon Kayaku, Novartis, Sun Pharmaceutical Industries, Pfizer, Taiho Pharmaceutical, Teijin Limited, Torii Pharmaceutical, Tsumura & Co. and UCB Pharma. Norito Katoh has received honoraria as a speaker/consultant for Sanofi, Maruho, Abbvie, Ely‐Lilly Japan, Leo Pharma, Jansen Pharma, Mitsubishi Tanabe Pharma, Kyowa Kirin, Celgene Japan and has received grants as an investigator from Maruho, Ely‐Lilly Japan, Sun Pharma, Taiho Pharmaceutical, Torii Pharmaceutical, Boehringer Ingelheim Japan Mitsubishi Tanabe Pharma, Kyowa Kirin, and Leo Pharma. Kyoko Ikumi, Aya Yamamoto, Haruna Nishihara, Yukihiko Watanabe and Ayano Maruyama have nothing to disclose.
Figures



References
-
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. - PubMed
-
- Kelly JB 3rd, Foley P, Strober BE. Current and future oral systemic therapies for psoriasis. Dermatol Clin. 2015;33:91–109. - PubMed
-
- Morita A. Current developments in phototherapy for psoriasis. J Dermatol. 2018;45:287–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

