Development and validation of novel sepsis subphenotypes using trajectories of vital signs
- PMID: 36152041
- PMCID: PMC9510534
- DOI: 10.1007/s00134-022-06890-z
Development and validation of novel sepsis subphenotypes using trajectories of vital signs
Abstract
Purpose: Sepsis is a heterogeneous syndrome and identification of sub-phenotypes is essential. This study used trajectories of vital signs to develop and validate sub-phenotypes and investigated the interaction of sub-phenotypes with treatment using randomized controlled trial data.
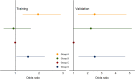
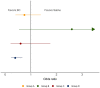
Methods: All patients with suspected infection admitted to four academic hospitals in Emory Healthcare between 2014-2017 (training cohort) and 2018-2019 (validation cohort) were included. Group-based trajectory modeling was applied to vital signs from the first 8 h of hospitalization to develop and validate vitals trajectory sub-phenotypes. The associations between sub-phenotypes and outcomes were evaluated in patients with sepsis. The interaction between sub-phenotype and treatment with balanced crystalloids versus saline was tested in a secondary analysis of SMART (Isotonic Solutions and Major Adverse Renal Events Trial).
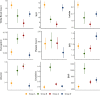
Results: There were 12,473 patients with suspected infection in training and 8256 patients in validation cohorts, and 4 vitals trajectory sub-phenotypes were found. Group A (N = 3483, 28%) were hyperthermic, tachycardic, tachypneic, and hypotensive. Group B (N = 1578, 13%) were hyperthermic, tachycardic, tachypneic (not as pronounced as Group A) and hypertensive. Groups C (N = 4044, 32%) and D (N = 3368, 27%) had lower temperatures, heart rates, and respiratory rates, with Group C normotensive and Group D hypotensive. In the 6,919 patients with sepsis, Groups A and B were younger while Groups C and D were older. Group A had the lowest prevalence of congestive heart failure, hypertension, diabetes mellitus, and chronic kidney disease, while Group B had the highest prevalence. Groups A and D had the highest vasopressor use (p < 0.001 for all analyses above). In logistic regression, 30-day mortality was significantly higher in Groups A and D (p < 0.001 and p = 0.03, respectively). In the SMART trial, sub-phenotype significantly modified treatment effect (p = 0.03). Group D had significantly lower odds of mortality with balanced crystalloids compared to saline (odds ratio (OR) 0.39, 95% confidence interval (CI) 0.23-0.67, p < 0.001).
Conclusion: Sepsis sub-phenotypes based on vital sign trajectory were consistent across cohorts, had distinct outcomes, and different responses to treatment with balanced crystalloids versus saline.
Keywords: Intravenous fluids; Phenotypes; Sepsis; Sub-phenotypes; Vital signs.
© 2022. Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
SVB is supported by the ATS-GSK Research Grant in COVID-19 and by NIH/NIGMS K23GM144867. MMC is supported by NIGMS (R01GM123193), Department of Defense (W81XWH-21-1-0009), NIA (R21 AG068720), and NIAAA (R01 DA051464-01). MMC has a patent pending (ARCD. P0535US.P2) for risk stratification algorithms for hospitalized patients and has received research support from EarlySense (Tel Aviv, Israel). CMC is supported by funding from the National Institutes of Health (GM072808, GM104323, AA027396). ETQ was supported by NHLBI T32HL087738. CR was supported by NCATS UL1TR002378.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

