Ubiquitin-like protein FAT10 promotes renal fibrosis by stabilizing USP7 to prolong CHK1-mediated G2/M arrest in renal tubular epithelial cells
- PMID: 36152057
- PMCID: PMC9550257
- DOI: 10.18632/aging.204301
Ubiquitin-like protein FAT10 promotes renal fibrosis by stabilizing USP7 to prolong CHK1-mediated G2/M arrest in renal tubular epithelial cells
Abstract
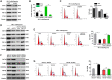
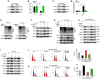
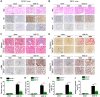
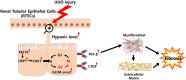
Renal fibrosis is the pathological hallmark of chronic kidney disease that is influenced by numerous factors. Arrest of renal tubular epithelial cells (RTECs) in G2/M phase is closely correlated with the progression of renal fibrosis; however, the mechanisms mediating these responses remain poorly defined. In this study, we observed that human leukocyte antigen-F adjacent transcript 10 (FAT10) deficiency abolished hypoxia-induced upregulation of checkpoint kinase 1 (CHK1) expression in RTECs derived from FAT10+/+ and FAT10-/- mice. Further investigations revealed that FAT10 contributes to CHK1-mediated G2/M arrest and production of pro-fibrotic cytokines in RTECs exposed to hypoxia. Mechanistically, FAT10 directly interacted with and stabilized the deubiquitylating enzyme ubiquitin specific protease 7 (USP7) to mediate CHK1 upregulation, thereby promoting CHK1-mediated G2/M arrest in RTECs. In animal model, FAT10 expression was upregulated in the obstructed kidneys of mice induced by unilateral ureteric obstruction injury, and FAT10-/- mice exhibited reduced unilateral ureteric obstruction injury induced-renal fibrosis compared with FAT10+/+ mice. Furthermore, in a cohort of patients with calculi-related chronic kidney disease, upregulated FAT10 expression was positively correlated with renal fibrosis and the USP7/CHK1 axis. These novel findings indicate that FAT10 prolongs CHK1-mediated G2/M arrest via USP7 to promote renal fibrosis, and inhibition of the FAT10/USP7/CHK1 axis might be a plausible therapeutic approach to alleviate renal fibrosis in chronic kidney disease.
Keywords: FAT10; cell cycle (G2/M) arrest; checkpoint kinase 1; renal fibrosis; ubiquitin specific protease 7.
Conflict of interest statement
Figures



Similar articles
-
Downregulation of fatty acid oxidation led by Hilpda increases G2/M arrest/delay-induced kidney fibrosis.Biochim Biophys Acta Mol Basis Dis. 2023 Jun;1869(5):166701. doi: 10.1016/j.bbadis.2023.166701. Epub 2023 Mar 28. Biochim Biophys Acta Mol Basis Dis. 2023. PMID: 36990128
-
HDAC9-mediated epithelial cell cycle arrest in G2/M contributes to kidney fibrosis in male mice.Nat Commun. 2023 May 25;14(1):3007. doi: 10.1038/s41467-023-38771-4. Nat Commun. 2023. PMID: 37230975 Free PMC article.
-
The ubiquitin-like protein FAT10 mediates NF-kappaB activation.J Am Soc Nephrol. 2010 Feb;21(2):316-26. doi: 10.1681/ASN.2009050479. Epub 2009 Dec 3. J Am Soc Nephrol. 2010. PMID: 19959714 Free PMC article.
-
Ubiquitin-like protein FAT10: A potential cardioprotective factor and novel therapeutic target in cancer.Clin Chim Acta. 2020 Nov;510:802-811. doi: 10.1016/j.cca.2020.09.016. Epub 2020 Sep 16. Clin Chim Acta. 2020. PMID: 32946796 Review.
-
Regulation of Interferon Induction by the Ubiquitin-Like Modifier FAT10.Biomolecules. 2020 Jun 23;10(6):951. doi: 10.3390/biom10060951. Biomolecules. 2020. PMID: 32586037 Free PMC article. Review.
Cited by
-
Crosstalk of ubiquitin system and non-coding RNA in fibrosis.Int J Biol Sci. 2024 Jul 8;20(10):3802-3822. doi: 10.7150/ijbs.93644. eCollection 2024. Int J Biol Sci. 2024. PMID: 39113708 Free PMC article. Review.
-
Renal tubular epithelial cell quality control mechanisms as therapeutic targets in renal fibrosis.J Pharm Anal. 2024 Aug;14(8):100933. doi: 10.1016/j.jpha.2024.01.001. Epub 2024 Jan 3. J Pharm Anal. 2024. PMID: 39247486 Free PMC article. Review.
-
The roles of the ubiquitin-proteasome system in renal disease.Int J Med Sci. 2025 Mar 10;22(8):1791-1810. doi: 10.7150/ijms.107284. eCollection 2025. Int J Med Sci. 2025. PMID: 40225869 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

