Associations between near end-of-life flortaucipir PET and postmortem CTE-related tau neuropathology in six former American football players
- PMID: 36152064
- PMCID: PMC9816291
- DOI: 10.1007/s00259-022-05963-x
Associations between near end-of-life flortaucipir PET and postmortem CTE-related tau neuropathology in six former American football players
Abstract
Purpose: Flourine-18-flortaucipir tau positron emission tomography (PET) was developed for the detection for Alzheimer's disease. Human imaging studies have begun to investigate its use in chronic traumatic encephalopathy (CTE). Flortaucipir-PET to autopsy correlation studies in CTE are needed for diagnostic validation. We examined the association between end-of-life flortaucipir PET and postmortem neuropathological measurements of CTE-related tau in six former American football players.
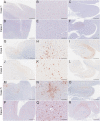
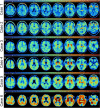
Methods: Three former National Football League players and three former college football players who were part of the DIAGNOSE CTE Research Project died and agreed to have their brains donated. The six players had flortaucipir (tau) and florbetapir (amyloid) PET prior to death. All brains from the deceased participants were neuropathologically evaluated for the presence of CTE. On average, the participants were 59.0 (SD = 9.32) years of age at time of PET. PET scans were acquired 20.33 (SD = 13.08) months before their death. Using Spearman correlation analyses, we compared flortaucipir standard uptake value ratios (SUVRs) to digital slide-based AT8 phosphorylated tau (p-tau) density in a priori selected composite cortical, composite limbic, and thalamic regions-of-interest (ROIs).
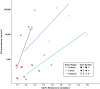
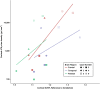
Results: Four brain donors had autopsy-confirmed CTE, all with high stage disease (n = 3 stage III, n = 1 stage IV). Three of these four met criteria for the clinical syndrome of CTE, known as traumatic encephalopathy syndrome (TES). Two did not have CTE at autopsy and one of these met criteria for TES. Concomitant pathology was only present in one of the non-CTE cases (Lewy body) and one of the CTE cases (motor neuron disease). There was a strong association between flortaucipir SUVRs and p-tau density in the composite cortical (ρ = 0.71) and limbic (ρ = 0.77) ROIs. Although there was a strong association in the thalamic ROI (ρ = 0.83), this is a region with known off-target binding. SUVRs were modest and CTE and non-CTE cases had overlapping SUVRs and discordant p-tau density for some regions.
Conclusions: Flortaucipir-PET could be useful for detecting high stage CTE neuropathology, but specificity to CTE p-tau is uncertain. Off-target flortaucipir binding in the hippocampus and thalamus complicates interpretation of these associations. In vivo biomarkers that can detect the specific p-tau of CTE across the disease continuum are needed.
Keywords: Biomarkers; Chronic traumatic encephalopathy; Flortaucipir; Football; Neurodegenerative disease; Positron emission tomography imaging; Repetitive head impacts; Tau.
© 2022. The Author(s).
Conflict of interest statement
CHA consulted for Avion, CND Life Sciences, Jazz, and Precon Health. LJB is Editor-in-Chief of the
Figures
Comment on
-
Tau PET and multimodal brain imaging in patients at risk for chronic traumatic encephalopathy.Neuroimage Clin. 2019;24:102025. doi: 10.1016/j.nicl.2019.102025. Epub 2019 Oct 17. Neuroimage Clin. 2019. PMID: 31670152 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- P20 AG068053/AG/NIA NIH HHS/United States
- R01NS078337/NS/NINDS NIH HHS/United States
- 1UL1TR001430/TR/NCATS NIH HHS/United States
- K23 NS102399/NS/NINDS NIH HHS/United States
- P20AG068053/AG/NIA NIH HHS/United States
- IK2 BX004349/BX/BLRD VA/United States
- P20GM109025/GM/NIGMS NIH HHS/United States
- P20 GM109025/GM/NIGMS NIH HHS/United States
- R01 AG053798/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- P30AG072980/AG/NIA NIH HHS/United States
- R01AG053798/AG/NIA NIH HHS/United States
- R35 AG071476/AG/NIA NIH HHS/United States
- P30 AG066512/AG/NIA NIH HHS/United States
- U54NS115266/NS/NINDS NIH HHS/United States
- U01NS093334/NS/NINDS NIH HHS/United States
- RF1NS122854/NS/NINDS NIH HHS/United States
- U01 NS093334/NS/NINDS NIH HHS/United States
- R01 NS100952/NS/NINDS NIH HHS/United States
- P30 AG072980/AG/NIA NIH HHS/United States
- P30AG019610/AG/NIA NIH HHS/United States
- P30AG072978/AG/NIA NIH HHS/United States
- K23NS102399/NS/NINDS NIH HHS/United States
- R01NS100952/NS/NINDS NIH HHS/United States
- R35AG71476/AG/NIA NIH HHS/United States
- UL1 TR001430/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

