Updating our understanding of health-related quality of life issues in children with cancer: a systematic review of patient-reported outcome measures and qualitative studies
- PMID: 36152110
- PMCID: PMC9510324
- DOI: 10.1007/s11136-022-03259-z
Updating our understanding of health-related quality of life issues in children with cancer: a systematic review of patient-reported outcome measures and qualitative studies
Abstract
Background: Health-related quality of life (HRQOL) is a key concept in pediatric oncology. This systematic review aims to update the conceptual HRQOL model by Anthony et al. (Qual Life Res 23(3):771-789, 2014), covering physical, emotional, social and general HRQOL aspects, and to present a comprehensive overview of age- and disease-specific HRQOL issues in children with cancer.
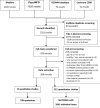
Methods: Medline, PsychINFO, the Cochrane Database for Systematic Reviews (CDSR), and the COSMIN database were searched (up to 31.12.2020) for publications using patient-reported outcome measures (PROMs) and qualitative studies in children with cancer (8-14-year) or their parents. Items and quotations were extracted and mapped onto the conceptual model for HRQOL in children with cancer mentioned above.
Results: Of 2038 identified studies, 221 were included for data extraction. We identified 96 PROMS with 2641 items and extracted 798 quotations from 45 qualitative studies. Most items and quotations (94.8%) could be mapped onto the conceptual model. However, some adaptations were made and the model was complemented by (sub)domains for 'treatment burden', 'treatment involvement', and 'financial issues'. Physical and psychological aspects were more frequently covered than social issues.
Discussion: This review provides a comprehensive overview of HRQOL issues for children with cancer. Our findings mostly support the HRQOL model by Anthony et al. (Qual Life Res 23(3):771-789, 2014), but some adaptations are suggested. This review may be considered a starting point for a refinement of our understanding of HRQOL in children with cancer. Further qualitative research will help to evaluate the comprehensiveness of the HRQOL model and the relevance of the issues it encompasses.
Keywords: Children with cancer; Concept elicitation; Health-related quality of life; Pediatric oncology; Quality of life.
© 2022. The Author(s).
Conflict of interest statement
The authors have no relevant conflicts of interest to disclose.
Figures
Similar articles
-
A critical evaluation of the content validity of patient-reported outcome measures assessing health-related quality of life in children with cancer: a systematic review.J Patient Rep Outcomes. 2023 Jan 19;7(1):2. doi: 10.1186/s41687-023-00540-8. J Patient Rep Outcomes. 2023. PMID: 36656407 Free PMC article.
-
Factors that influence parents' and informal caregivers' views and practices regarding routine childhood vaccination: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2021 Oct 27;10(10):CD013265. doi: 10.1002/14651858.CD013265.pub2. Cochrane Database Syst Rev. 2021. PMID: 34706066 Free PMC article.
-
A systematic review and economic evaluation of epoetin alpha, epoetin beta and darbepoetin alpha in anaemia associated with cancer, especially that attributable to cancer treatment.Health Technol Assess. 2007 Apr;11(13):1-202, iii-iv. doi: 10.3310/hta11130. Health Technol Assess. 2007. PMID: 17408534
-
Inhaled mannitol for cystic fibrosis.Cochrane Database Syst Rev. 2018 Feb 9;2(2):CD008649. doi: 10.1002/14651858.CD008649.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 May 1;5:CD008649. doi: 10.1002/14651858.CD008649.pub4. PMID: 29424930 Free PMC article. Updated.
-
Peer support interventions for parents and carers of children with complex needs.Cochrane Database Syst Rev. 2021 Dec 20;12(12):CD010618. doi: 10.1002/14651858.CD010618.pub2. Cochrane Database Syst Rev. 2021. PMID: 34923624 Free PMC article.
Cited by
-
"I'm learning to live after cancer and its treatment": Exploring the challenges of cancer survivorship in Arkansas.SSM Qual Res Health. 2025 Jun;7:100519. doi: 10.1016/j.ssmqr.2024.100519. Epub 2024 Dec 19. SSM Qual Res Health. 2025. PMID: 40575724 Free PMC article.
-
The quality of life assessment in children with juvenile idiopathic arthritis- comparison of PROMIS® generic and disease-specific cut-off points: a pilot study.Rheumatol Int. 2025 Feb 24;45(3):61. doi: 10.1007/s00296-025-05797-4. Rheumatol Int. 2025. PMID: 39992379
-
A critical evaluation of the content validity of patient-reported outcome measures assessing health-related quality of life in children with cancer: a systematic review.J Patient Rep Outcomes. 2023 Jan 19;7(1):2. doi: 10.1186/s41687-023-00540-8. J Patient Rep Outcomes. 2023. PMID: 36656407 Free PMC article.
-
Consensus framework for conducting phase I/II clinical trials for children, adolescents, and young adults with pediatric low-grade glioma: Guidelines established by the International Pediatric Low-Grade Glioma Coalition Clinical Trial Working Group.Neuro Oncol. 2024 Mar 4;26(3):407-416. doi: 10.1093/neuonc/noad227. Neuro Oncol. 2024. PMID: 38146999 Free PMC article.
-
Association of Socioeconomic Factors and Physical Activity with Health-Related Quality of Life in Italian Middle School Children: An Exploratory Cross-Sectional Study.Healthcare (Basel). 2023 Jul 22;11(14):2092. doi: 10.3390/healthcare11142092. Healthcare (Basel). 2023. PMID: 37510533 Free PMC article.
References
-
- Howlader N, Noone A, Krapcho M, Miller D, Brest A, Yu M, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER cancer statistics review, 1975–2018. National Cancer Institute; 2021.
-
- Institute NC. Children with cancer—A guide for parents. National Institutes of Health; 2015.
-
- Agency EM. Appendix 2 to the guideline on the evaluation of anticancer medicinal products in man: The use of patient-reported outcome (PRO) measures in oncology studies. European Medicines Agency; 2016.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical