Protection against omicron (B.1.1.529) BA.2 reinfection conferred by primary omicron BA.1 or pre-omicron SARS-CoV-2 infection among health-care workers with and without mRNA vaccination: a test-negative case-control study
- PMID: 36152671
- PMCID: PMC9491856
- DOI: 10.1016/S1473-3099(22)00578-3
Protection against omicron (B.1.1.529) BA.2 reinfection conferred by primary omicron BA.1 or pre-omicron SARS-CoV-2 infection among health-care workers with and without mRNA vaccination: a test-negative case-control study
Abstract
Background: There is a paucity of data on vaccine-induced or infection-induced (hybrid or natural) immunity against omicron (B.1.1.529) subvariant BA.2, particularly in comparing the effects of previous SARS-CoV-2 infection with the same or different genetic lineage. We aimed to estimate the protection against omicron BA.2 associated with previous primary infection with omicron BA.1 or pre-omicron SARS-CoV-2, among health-care workers with and without mRNA vaccination.
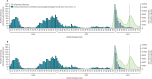
Methods: We conducted a test-negative case-control study among health-care workers aged 18 years or older who were tested for SARS-CoV-2 in Quebec, Canada, between March 27 and June 4, 2022, when BA.2 was the predominant variant and was presumptively diagnosed with a positive test result. We identified cases (positive test during study period) and controls (negative test during study period) using the provincial laboratory database that records all nucleic acid amplification testing for SARS-CoV-2 in Quebec, and used the provincial immunisation registry to determine vaccination status. Logistic regression models compared the likelihood of BA.2 infection or reinfection (second positive test ≥30 days after primary infection) among health-care workers who had previous primary infection and none to three mRNA vaccine doses versus unvaccinated health-care workers with no primary infection.
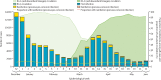
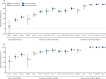
Findings: 258 007 SARS-CoV-2 tests were done during the study period. Among those with a valid result and that met the inclusion criteria, there were 37 732 presumed BA.2 cases (2521 [6·7%] reinfections following pre-omicron primary infection and 659 [1·7%] reinfections following BA.1 primary infection) and 73 507 controls (7360 [10·0%] had pre-omicron primary infection and 12 315 [16·8%] had BA.1 primary infection). Pre-omicron primary infection was associated with a 38% (95% CI 19-53) reduction in BA.2 infection risk, with higher BA.2 protection among those who had also received one (56%, 95% CI 47-63), two (69%, 64-73), or three (70%, 66-74) mRNA vaccine doses. Omicron BA.1 primary infection was associated with greater protection against BA.2 infection (risk reduction of 72%, 95% CI 65-78), and protection was increased further among those who had received two doses of mRNA vaccine (96%, 95-96), but was not improved with a third dose (96%, 95-97).
Interpretation: Health-care workers who had received two doses of mRNA vaccine and had previous BA.1 infection were subsequently well protected for a prolonged period against BA.2 reinfection, with a third vaccine dose conferring no improvement to that hybrid protection. If this protection also pertains to future variants, there might be limited benefit from additional vaccine doses for people with hybrid immunity, depending on timing and variant.
Funding: Ministère de la Santé et des Services Sociaux du Québec.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests SC, MO, and GDS report financial support from the Ministère de la Santé et des Services Sociaux du Québec to their institution for this work, during the conduct of the study. GDS reports a grant from Pfizer for a “Meningococcal B antibody seroprevalence study”, outside of the submitted work. RG reports personal fees from AbbVie honorary for a conference on respiratory syncytial virus burden in children, outside of the submitted work. DT is supported by a research career award from the Fonds de Recherche du Québec–Santé. JF reports grants from the Ministry of Health of Quebec for sequencing of SARS-CoV-2 positive samples and grants from Cancogen (Génome Canada) for sequencing of SARS-CoV-2-positive samples, outside of the submitted work; and is chair of the provincial genomic surveillance committee of SARS-CoV-2 (INSPQ, Quebec). DMS reports grants paid to their institution from Public Health Agency of Canada, Michael Smith Foundation for Health Research, Canadian Institutes of Health Research, and BCCDC Foundation for Public Health, outside of the submitted work. All other authors declare no competing interests.
Figures
Comment in
-
Hybrid immunity and strategies for COVID-19 vaccination.Lancet Infect Dis. 2023 Jan;23(1):2-3. doi: 10.1016/S1473-3099(22)00640-5. Epub 2022 Sep 21. Lancet Infect Dis. 2023. PMID: 36152670 Free PMC article. No abstract available.
References
-
- Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the omicron variant of SARS-CoV-2. Nature. 2022;602:676–681. - PubMed
-
- WHO Statement on omicron sublineage BA.2. 2022. https://www.who.int/news/item/22-02-2022-statement-on-omicron-sublineage...
-
- Government of Canada COVID-19 daily epidemiological update. Coronavirus disease (COVID-19) 2022. https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid...
-
- Institut National de Santé Publique du Québec Données sur les variants du SRAS-CoV-2 au Québec. 2022. https://www.inspq.qc.ca/covid-19/donnees/variants
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

