Does repeated influenza vaccination attenuate effectiveness? A systematic review and meta-analysis
- PMID: 36152673
- PMCID: PMC9780123
- DOI: 10.1016/S2213-2600(22)00266-1
Does repeated influenza vaccination attenuate effectiveness? A systematic review and meta-analysis
Abstract
Background: Influenza vaccines require annual readministration; however, several reports have suggested that repeated vaccination might attenuate the vaccine's effectiveness. We aimed to estimate the reduction in vaccine effectiveness associated with repeated influenza vaccination.
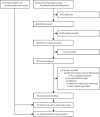
Methods: In this systematic review and meta-analysis, we searched MEDLINE, EMBASE, and CINAHL Complete databases for articles published from Jan 1, 2016, to June 13, 2022, and Web of Science for studies published from database inception to June 13, 2022. For studies published before Jan 1, 2016, we consulted published systematic reviews. Two reviewers (EJ-G and EJR) independently screened, extracted data using a data collection form, assessed studies' risk of bias using the Risk Of Bias In Non-Randomized Studies of Interventions (ROBINS-I) and evaluated the weight of evidence by Grading of Recommendations Assessment, Development, and Evaluation (GRADE). We included observational studies and randomised controlled trials that reported vaccine effectiveness against influenza A(H1N1)pdm09, influenza A(H3N2), or influenza B using four vaccination groups: current season; previous season; current and previous seasons; and neither season (reference). For each study, we calculated the absolute difference in vaccine effectiveness (ΔVE) for current season only and previous season only versus current and previous season vaccination to estimate attenuation associated with repeated vaccination. Pooled vaccine effectiveness and ∆VE were calculated by season, age group, and overall. This study is registered with PROSPERO, CRD42021260242.
Findings: We identified 4979 publications, selected 681 for full review, and included 83 in the systematic review and 41 in meta-analyses. ΔVE for vaccination in both seasons compared with the current season was -9% (95% CI -16 to -1, I2=0%; low certainty) for influenza A(H1N1)pdm09, -18% (-26 to -11, I2=7%; low certainty) for influenza A(H3N2), and -7% (-14 to 0, I2=0%; low certainty) for influenza B, indicating lower protection with consecutive vaccination. However, for all types, A subtypes and B lineages, vaccination in both seasons afforded better protection than not being vaccinated.
Interpretation: Our estimates suggest that, although vaccination in the previous year attenuates vaccine effectiveness, vaccination in two consecutive years provides better protection than does no vaccination. The estimated effects of vaccination in the previous year are concerning and warrant additional investigation, but are not consistent or severe enough to support an alternative vaccination regimen at this time.
Funding: WHO and the US National Institutes of Health.
Copyright © 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests SGS and AF report the following funding for influenza vaccination and infection studies: US National Institutes of Health (SGS and AF); US Centers for Disease Control (SGS and AF); the National Health and Medical Research Council of Australia (AF); and OptumLabs research credits (SGS). SGS and AF are employed by the WHO Collaborating Centre for Reference and Research on Influenza, which receives funding from the International Federation of Pharmaceutical Manufacturers and Associations and Seqiris for the development of influenza vaccines. SGS has served in an unpaid capacity on advisory boards for Sanofi and Seqiris. From 2017–21, SGS was a member of the WHO Strategic Advisory Group of Experts (SAGE) on Immunization Working Group on Influenza. SGS serves on the Australian National Influenza Surveillance Committee. EJ-G, EJR, and AJK declare no competing interests.
Figures



Comment in
-
Repeat vaccination and influenza vaccine effectiveness.Lancet Respir Med. 2023 Jan;11(1):2-3. doi: 10.1016/S2213-2600(22)00305-8. Epub 2022 Sep 21. Lancet Respir Med. 2023. PMID: 36152672 No abstract available.
References
-
- Kissling E, Nunes B, Robertson C, et al. I-MOVE multicentre case-control study 2010/11 to 2014/15: is there within-season waning of influenza type/subtype vaccine effectiveness with increasing time since vaccination? Euro Surveill. 2016;21:13–24. - PubMed
-
- Ferdinands JM, Fry AM, Reynolds S, et al. Intraseason waning of influenza vaccine protection: evidence from the US Influenza Vaccine Effectiveness Network, 2011–12 through 2014–15. Clin Infect Dis. 2017;64:544–550. - PubMed
-
- Vaccines against influenza WHO position paper—November 2012. Wkly Epidemiol Rec. 2012;87:461–476. - PubMed
-
- Hoskins TW, Davies JR, Smith AJ, Miller CL, Allchin A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ's Hospital. Lancet. 1979;1:33–35. - PubMed

