Assessing the roles of collagen fiber morphology and matrix stiffness on ovarian cancer cell migration dynamics using multiphoton fabricated orthogonal image-based models
- PMID: 36152908
- PMCID: PMC10324295
- DOI: 10.1016/j.actbio.2022.09.037
Assessing the roles of collagen fiber morphology and matrix stiffness on ovarian cancer cell migration dynamics using multiphoton fabricated orthogonal image-based models
Abstract
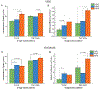
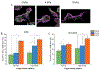
Ovarian cancer remains the deadliest of the gynecological cancers, where this arises from poor screening and imaging tools that can detect early disease, and also limited understanding of the structural and functional aspects of the tumor microenvironment. To gain insight into the underlying cellular dynamics, we have used multiphoton excited fabrication to create Second Harmonic Generation (SHG) image-based orthogonal models from collagen/GelMA that represent both the collagen matrix morphology and stiffness (∼2-8 kPa) of normal ovarian stroma and high grade serous ovarian cancers (HGSOC). These scaffolds are used to study migration/cytoskeletal dynamics of normal (IOSE) and ovarian cancer (OVCA433) cell lines. We found that the highly aligned fiber morphology of HGSOC promotes aspects of motility (motility coefficient, motility, and focal adhesion expression) through a contact guidance mechanism and that stiffer matrix further promotes these same processes through a mechanosensitive mechanism, where these trends were similar for both normal and cancer cells. However, cell specific differences were found on these orthogonal models relative to those providing only morphology, showing the importance of presenting both morphology and stiffness cues. Moreover, we found increased cadherin expression and decreased cell alignment only for cancer cells on scaffolds of intermediate modulus suggesting different stiffness-dependent mechanotransduction mechanisms are engaged. This overall approach affords decoupling the roles of matrix morphology, stiffness and cell genotype and affords hypothesis testing of the factors giving rise to disease progression and metastasis. Further, more established fabrication techniques cannot simultaneously reproduce both the 3D collagen fiber morphology and stiffness. STATEMENT OF SIGNIFICANCE: Ovarian cancer metastasizes when lesions are small, where cells exfoliate from the surface of the ovary and reattach at distal sites in the peritoneum. The adhesion/migration dynamics are not well understood and there is a need for new 3D in vitro models of the extracellular matrix to study the biology. Here we use multiphoton excited crosslinking to fabricate ECM orthogonal models that represent the collagen morphology and stiffness in human ovarian tissues. These are then used to study ovarian cancer cell migration dynamics and we found that contact guidance and a mechanosensitive response and cell genotype all combine to affect the behavior. These models provide insight into disease etiology and progression not readily possible by other fabrication methods.
Keywords: Collagen; Extracellular matrix; Fabrication; Migration; Tumor microenvironment.
Copyright © 2022 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no competing interests.
Figures


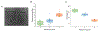



References
-
- Cancer Facts & Figures 2018. American Cancer Society (ACS), (2018).
-
- Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Cancer Statistics Review, 1975-2014-SEER Statistics, National Cancer Institute, (2016).
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A, Cancer statistics, 2021, 71(1) (2021) 7–33. - PubMed
-
- I. National Cancer, SEER Cancer Stat Facts: ovarian cancer, (2018).
-
- Karlan BY, The status of ultrasound and color Doppler imaging for the early detection of ovarian carcinoma, Cancer Invest 15(3) (1997) 265–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

