Effects of a Novel Beta Lactam Compound, MC-100093, on the Expression of Glutamate Transporters/Receptors and Ethanol Drinking Behavior of Alcohol-Preferring Rats
- PMID: 36153003
- PMCID: PMC9667983
- DOI: 10.1124/jpet.122.001147
Effects of a Novel Beta Lactam Compound, MC-100093, on the Expression of Glutamate Transporters/Receptors and Ethanol Drinking Behavior of Alcohol-Preferring Rats
Abstract
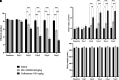
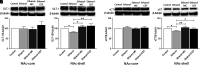
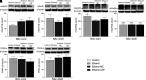
Chronic ethanol exposure affects the glutamatergic system in several brain reward regions including the nucleus accumbens (NAc). Our laboratory has shown that chronic exposure to ethanol reduced the expression of glutamate transporter 1 (GLT-1) and cystine/glutamate exchanger (xCT) and, as a result, increased extracellular glutamate concentrations in the NAc of alcohol-preferring (P) rats. Moreover, previous studies from our laboratory reported that chronic ethanol intake altered the expression of certain metabotropic glutamate receptors in the brain. In addition to central effects, chronic ethanol consumption induced liver injury, which is associated with steatohepatitis. In the present study, we investigated the effects of chronic ethanol consumption in the brain and liver. Male P rats had access to a free choice of ethanol and water bottles for five weeks. Chronic ethanol consumption reduced GLT-1 and xCT expression in the NAc shell but not in the NAc core. Furthermore, chronic ethanol consumption increased fat droplet content as well as peroxisome proliferator-activated receptor alpha (PPAR-α) and GLT-1 expression in the liver. Importantly, treatment with the novel beta-lactam compound, MC-100093, reduced ethanol drinking behavior and normalized the levels of GLT-1 and xCT expression in the NAc shell as well as normalized GLT-1 and PPAR-α expression in the liver. In addition, MC-100093 attenuated ethanol-induced increases in fat droplet content in the liver. These findings suggest that MC-100093 may be a potential lead compound to attenuate ethanol-induced dysfunction in the glutamatergic system and liver injury. SIGNIFICANCE STATEMENT: This study identified a novel beta-lactam, MC-100093, that has demonstrated upregulatory effects on GLT-1. MC-100093 reduced ethanol drinking behavior and normalized levels of GLT-1 and xCT expression in the NAc shell as well as normalized GLT-1 and PPAR-α expression in the liver. In addition, MC-100093 attenuated ethanol-induced increases in fat droplet content in the liver.
Copyright © 2022 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


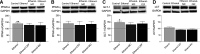
References
-
- Abou-Gharbia M, Blass B, Childers W, Ramanjulu M, Melenski E (2017) Glutamate transporter-1 (GLT-1): a potential therapeutic target for the treatment of central nervous system diseases and disorders. Drugs Fut 42:489–509.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

