Safety and immune response kinetics of GRAd-COV2 vaccine: phase 1 clinical trial results
- PMID: 36153335
- PMCID: PMC9509317
- DOI: 10.1038/s41541-022-00531-8
Safety and immune response kinetics of GRAd-COV2 vaccine: phase 1 clinical trial results
Abstract
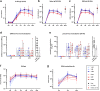
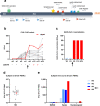
Despite the successful deployment of efficacious vaccines and therapeutics, the development of novel vaccines for SARS-CoV-2 remains a major goal to increase vaccine doses availability and accessibility for lower income setting. We report here on the kinetics of Spike-specific humoral and T-cell response in young and old volunteers over 6 months follow-up after a single intramuscular administration of GRAd-COV2, a gorilla adenoviral vector-based vaccine candidate currently in phase-2 of clinical development. At all three tested vaccine dosages, Spike binding and neutralizing antibodies were induced and substantially maintained up to 3 months, to then contract at 6 months. Potent T-cell responses were readily induced and sustained throughout the study period, with only minor decline. No major differences in immune response to GRAd-COV2 vaccination were observed in the two age cohorts. In light of its favorable safety and immunogenicity, GRAd-COV2 is a valuable candidate for further clinical development and potential addition to the COVID-19 vaccine toolbox to help fighting SARS-CoV-2 pandemic.
© 2022. The Author(s).
Conflict of interest statement
SB, A. Sommella, AMC, FB, FC, MG, AR, R. Camerini, S. Colloca, AF and SC are employees of ReiThera Srl. S. Colloca and AF are also shareholders of Keires AG, S. Colloca and AR are named inventor of the Patent Application No. 20183515.4 titled “GORILLA ADENOVIRUS NUCLEIC ACID- AND AMINO ACID-SEQUENCES, VECTORS CONTAINING SAME, AND USES THEREOF”. All remaining authors declare that they have no competing interests.
Figures
References
-
- WHO COVID-19 Dashboard-https://covid19.who.int/. (Last accessed on 28 Jan 2022).
Grants and funding
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
- Ricerca corrente line1/Ministry of Health, Italy | Agenzia Italiana del Farmaco, Ministero della Salute (Italian Medicines Agency)
LinkOut - more resources
Full Text Sources
Miscellaneous

