Dithranol as novel co-adjuvant for non-invasive dermal vaccination
- PMID: 36153349
- PMCID: PMC9509335
- DOI: 10.1038/s41541-022-00530-9
Dithranol as novel co-adjuvant for non-invasive dermal vaccination
Erratum in
-
Author Correction: Dithranol as novel co-adjuvant for non-invasive dermal vaccination.NPJ Vaccines. 2023 Jun 22;8(1):94. doi: 10.1038/s41541-023-00689-9. NPJ Vaccines. 2023. PMID: 37349361 Free PMC article. No abstract available.
Abstract
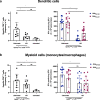
Transcutaneous immunization (TCI) utilizing the TLR7 agonist imiquimod (IMQ-TCI) induces T cell-driven protective immunity upon application onto intact skin. In our present work, we combine the anti-psoriatic agent dithranol with IMQ-TCI to boost vaccination efficacy (Dithranol/IMQ-based transcutaneous vaccination (DIVA)). Using ovalbumin-derived peptides as model antigens in mice, DIVA induced superior cytolytic CD8+ T cells and CD4+ T cells with a TH1 cytokine profile in the priming as well as in the memory phase. Regarding the underlying mechanisms, dithranol induced an oxidant-dependent, monocyte-attracting inflammatory milieu in the skin boosting TLR7-dependent activation of dendritic cells and macrophages leading to superior T cell priming and protective immunity in vaccinia virus infection. In conclusion, we introduce the non-invasive vaccination method DIVA to induce strong primary and memory T cell responses upon a single local treatment. This work provides relevant insights in cutaneous vaccination approaches, paving the way for clinical development in humans.
© 2022. The Author(s).
Conflict of interest statement
J.S., M.S., A.-K.H., and M.P.R. are inventors of a patent application submitted by the UMC Mainz (EP 18204287.9). The others authors declare that they have no competing interests.
Figures






References
-
- Lurie, N., Saville, M., Hatchett, R. & Halton, J. Developing Covid-19 vaccines at pandemic speed. N. Engl. J. Med.10.1056/nejmp2005630 (2020). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

