Recent findings on chimeric antigen receptor (CAR)-engineered immune cell therapy in solid tumors and hematological malignancies
- PMID: 36153626
- PMCID: PMC9509604
- DOI: 10.1186/s13287-022-03163-w
Recent findings on chimeric antigen receptor (CAR)-engineered immune cell therapy in solid tumors and hematological malignancies
Abstract
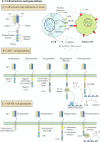
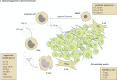
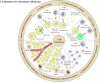
Advancements in adoptive cell therapy over the last four decades have revealed various new therapeutic strategies, such as chimeric antigen receptors (CARs), which are dedicated immune cells that are engineered and administered to eliminate cancer cells. In this context, CAR T-cells have shown significant promise in the treatment of hematological malignancies. However, many obstacles limit the efficacy of CAR T-cell therapy in both solid tumors and hematological malignancies. Consequently, CAR-NK and CAR-M cell therapies have recently emerged as novel therapeutic options for addressing the challenges associated with CAR T-cell therapies. Currently, many CAR immune cell trials are underway in various human malignancies around the world to improve antitumor activity and reduce the toxicity of CAR immune cell therapy. This review will describe the comprehensive literature of recent findings on CAR immune cell therapy in a wide range of human malignancies, as well as the challenges that have emerged in recent years.
Keywords: CAR T-cell; CAR-M solid tumors; CAR-NK cell; Chimeric antigen receptors; Hematological malignancies; Immunotherapy.
© 2022. The Author(s).
Conflict of interest statement
There is no conflict of interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

