Pharmacokinetics of Intramuscularly Administered Thermoresponsive Polymers
- PMID: 36153823
- PMCID: PMC11468617
- DOI: 10.1002/adhm.202201344
Pharmacokinetics of Intramuscularly Administered Thermoresponsive Polymers
Abstract
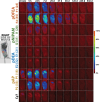
Aqueous solutions of some polymers exhibit a lower critical solution temperature (LCST); that is, they form phase-separated aggregates when heated above a threshold temperature. Such polymers found many promising (bio)medical applications, including in situ thermogelling with controlled drug release, polymer-supported radiotherapy (brachytherapy), immunotherapy, and wound dressing, among others. Yet, despite the extensive research on medicinal applications of thermoresponsive polymers, their biodistribution and fate after administration remained unknown. Thus, herein, they studied the pharmacokinetics of four different thermoresponsive polyacrylamides after intramuscular administration in mice. In vivo, these thermoresponsive polymers formed depots that subsequently dissolved with a two-phase kinetics (depot maturation, slow redissolution) with half-lives 2 weeks to 5 months, as depot vitrification prolonged their half-lives. Additionally, the decrease of TCP of a polymer solution increased the density of the intramuscular depot. Moreover, they detected secondary polymer depots in the kidneys and liver; these secondary depots also followed two-phase kinetics (depot maturation and slow dissolution), with half-lives 8 to 38 days (kidneys) and 15 to 22 days (liver). Overall, these findings may be used to tailor the properties of thermoresponsive polymers to meet the demands of their medicinal applications. Their methods may become a benchmark for future studies of polymer biodistribution.
Keywords: LCST; biodistribution; poly(2,2-difluoroethyl)acrylamide; poly(N,N-diethylacrylamide); poly(N-acryloylpyrolidine); poly(N-isopropylacrylamide); polyacrylamide; rational polymer design.
© 2022 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
O.G. is a stockholder of Bausch Health Companies Inc. (Laval, Canada), whose subsidiary licenses Visidic gel, which in turn was used in this study to prevent major side effects of anesthesia, such as keratitis sicca. The other authors have no conflict of interest to declare. No company, grant agency, or employer influenced the design of the study, its evaluation, or its conclusions.
Figures



References
-
- Aseyev V., Tenhu H., Winnik F. M., in Self Organized Nanostructures of Amphiphilic Block Copolymers II, (Eds: Müller A. H. E., Borisov O.), Springer, Berlin, Heidelberg: 2010, vol. 242, p. 29.
-
- Mano J. F., Adv. Eng. Mater. 2008, 10, 515.
-
- Chung J. E., Yokoyama M., Aoyagi T., Sakurai Y., Okano T., J. Controlled Release 1998, 53, 119. - PubMed
-
- Ward M. A., Georgiou T. K., Polymers 2011, 3, 1215.
-
- Schmaljohann D., Adv. Drug Delivery Rev. 2006, 58, 1655. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

