Discovery and Characterization of Synthesized and FDA-Approved Inhibitors of Clostridial and Bacillary Collagenases
- PMID: 36154055
- PMCID: PMC9574867
- DOI: 10.1021/acs.jmedchem.2c00785
Discovery and Characterization of Synthesized and FDA-Approved Inhibitors of Clostridial and Bacillary Collagenases
Abstract
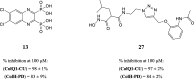
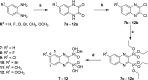
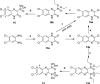
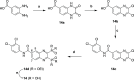
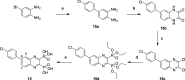
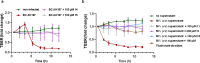
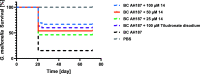
In view of the worldwide antimicrobial resistance (AMR) threat, new bacterial targets and anti-infective agents are needed. Since important roles in bacterial pathogenesis have been demonstrated for the collagenase H and G (ColH and ColG) from Clostridium histolyticum, collagenase Q1 and A (ColQ1 and ColA) from Bacillus cereus represent attractive antivirulence targets. Furthermore, repurposing FDA-approved drugs may assist to tackle the AMR crisis and was addressed in this work. Here, we report on the discovery of two potent and chemically stable bacterial collagenase inhibitors: synthesized and FDA-approved diphosphonates and hydroxamates. Both classes showed high in vitro activity against the clostridial and bacillary collagenases. The potent diphosphonates reduced B. cereus-mediated detachment and death of cells and Galleria mellonella larvae. The hydroxamates were also tested in a similar manner; they did not have an effect in infection models. This might be due to their fast binding kinetics to bacterial collagenases.
Conflict of interest statement
The authors declare the following competing financial interest(s): The authors declare the following financial interest(s): A.S.A, V.C., J.H., and A.K.H.H are co-inventors on an international patent application WO2022/043322 that incorporates compounds outlined in the library screening in the supplementary information.
Figures
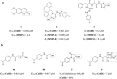




References
-
- Antibiotic resistance. https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance. (accessed Feb 13, 2022).
-
- No Time to Wait: Securing the Future from Drug-Resistant Infections. https://www.who.int/publications/i/item/no-time-to-wait-securing-the-fut.... (accessed Feb 13, 2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

