Bacterial Membrane Vesicles as a Novel Strategy for Extrusion of Antimicrobial Bismuth Drug in Helicobacter pylori
- PMID: 36154274
- PMCID: PMC9601102
- DOI: 10.1128/mbio.01633-22
Bacterial Membrane Vesicles as a Novel Strategy for Extrusion of Antimicrobial Bismuth Drug in Helicobacter pylori
Abstract
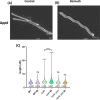
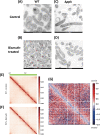
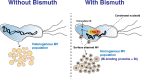
Bacterial antibiotic resistance is a major threat to human health. A combination of antibiotics with metals is among the proposed alternative treatments. Only one such combination is successfully used in clinics; it associates antibiotics with the metal bismuth to treat infections by Helicobacter pylori. This bacterial pathogen colonizes the human stomach and is associated with gastric cancer, killing 800,000 individuals yearly. The effect of bismuth in H. pylori treatment is not well understood in particular for sublethal doses such as those measured in the plasma of treated patients. We addressed this question and observed that bismuth induces the formation of homogeneously sized membrane vesicles (MVs) with unique protein cargo content enriched in bismuth-binding proteins, as shown by quantitative proteomics. Purified MVs of bismuth-exposed bacteria were strongly enriched in bismuth as measured by inductively coupled plasma optical emission spectrometry (ICP-OES), unlike bacterial cells from which they originate. Thus, our results revealed a novel function of MVs in bismuth detoxification, where secreted MVs act as tool to discard bismuth from the bacteria. Bismuth also induces the formation of intracellular polyphosphate granules that are associated with changes in nucleoid structure. Nucleoid compaction in response to bismuth was established by immunogold electron microscopy and refined by the first chromosome conformation capture (Hi-C) analysis of H. pylori. Our results reveal that even low doses of bismuth induce profound changes in H. pylori physiology and highlight a novel defense mechanism that involves MV-mediated bismuth extrusion from the bacteria and a probable local DNA protective response where polyphosphate granules are associated with nucleoid compaction. IMPORTANCE Bacterial resistance to antibiotics is a major threat to human health. Treatments combining antibiotics with metals were proposed to circumvent this hurdle. Only one such combination is successfully used in clinics associating antibiotics with the metal bismuth to treat infections by the human pathogen Helicobacter pylori. H. pylori causes 800,000 deaths by gastric cancer yearly. How bismuth impacts H. pylori and its response to this toxic metal were ill defined. We discovered that upon bismuth exposure, H. pylori secretes membrane vesicles that are enriched in bismuth. Bismuth also induces the formation of intracellular polyphosphate granules associated with compaction of the chromosome. Upon bismuth exposure, H. pylori displays both defense and protection mechanisms, with bismuth extrusion by vesicles and shielding of the chromosome.
Keywords: Helicobacter pylori; Hi-C; antibiotic resistance; bismuth; chromosome conformation capture; membrane vesicles; polyphosphate granules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Frei A, Zuegg J, Elliott AG, Baker M, Braese S, Brown C, Chen F, G Dowson C, Dujardin G, Jung N, King AP, Mansour AM, Massi M, Moat J, Mohamed HA, Renfrew AK, Rutledge PJ, Sadler PJ, Todd MH, Willans CE, Wilson JJ, Cooper MA, Blaskovich MAT. 2020. Metal complexes as a promising source for new antibiotics. Chem Sci 11:2627–2639. doi: 10.1039/c9sc06460e. - DOI - PMC - PubMed
-
- Tursi A, Franceschi M, Allegretta L, Savarino E, De Bastiani R, Elisei W, Baldassarre G, Ferronato A, Scida S, Miraglia C, Penna A, Licci C, Rizzo GL, Pranzo G, Cassieri C, Brandimarte G, Picchio M, Di Mario F. 2018. Effectiveness and safety of Pylera in patients infected by Helicobacter pylori: a multicenter, retrospective, real life study. Dig Dis 36:264–268. doi: 10.1159/000487391. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

