Canary in the Coal Mine: How Resistance Surveillance in Commensals Could Help Curb the Spread of AMR in Pathogenic Neisseria
- PMID: 36154280
- PMCID: PMC9600782
- DOI: 10.1128/mbio.01991-22
Canary in the Coal Mine: How Resistance Surveillance in Commensals Could Help Curb the Spread of AMR in Pathogenic Neisseria
Abstract
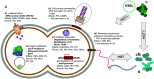
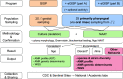
Antimicrobial resistance (AMR) is widespread within Neisseria gonorrhoeae populations. Recent work has highlighted the importance of commensal Neisseria (cN) as a source of AMR for their pathogenic relatives through horizontal gene transfer (HGT) of AMR alleles, such as mosaic penicillin binding protein 2 (penA), multiple transferable efflux pump (mtr), and DNA gyrase subunit A (gyrA) which impact beta-lactam, azithromycin, and ciprofloxacin susceptibility, respectively. However, nonpathogenic commensal species are rarely characterized. Here, we propose that surveillance of the universally carried commensal Neisseria may play the role of the "canary in the coal mine," and reveal circulating known and novel antimicrobial resistance determinants transferable to pathogenic Neisseria. We summarize the current understanding of commensal Neisseria as an AMR reservoir, and call to increase research on commensal Neisseria species, through expanding established gonococcal surveillance programs to include the collection, isolation, antimicrobial resistance phenotyping, and whole-genome sequencing (WGS) of commensal isolates. This will help combat AMR in the pathogenic Neisseria by: (i) determining the contemporary AMR profile of commensal Neisseria, (ii) correlating AMR phenotypes with known and novel genetic determinants, (iii) qualifying and quantifying horizontal gene transfer (HGT) for AMR determinants, and (iv) expanding commensal Neisseria genomic databases, perhaps leading to the identification of new drug and vaccine targets. The proposed modification to established Neisseria collection protocols could transform our ability to address AMR N. gonorrhoeae, while requiring minor modifications to current surveillance practices. IMPORTANCE Contemporary increases in the prevalence of antimicrobial resistance (AMR) in Neisseria gonorrhoeae populations is a direct threat to global public health and the effective treatment of gonorrhea. Substantial effort and financial support are being spent on identifying resistance mechanisms circulating within the gonococcal population. However, these surveys often overlook a known source of resistance for gonococci-the commensal Neisseria. Commensal Neisseria and pathogenic Neisseria frequently share DNA through horizontal gene transfer, which has played a large role in rendering antibiotic therapies ineffective in pathogenic Neisseria populations. Here, we propose the expansion of established gonococcal surveillance programs to integrate a collection, AMR profiling, and genomic sequencing pipeline for commensal species. This proposed expansion will enhance the field's ability to identify resistance in and from nonpathogenic reservoirs and anticipate AMR trends in pathogenic Neisseria.
Keywords: N. gonorrhoeae; Neisseria; Neisseria gonorrhoeae; antibiotic resistance; commensal bacteria; horizontal gene transfer; microbiome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Rowley J, Vander Hoorn S, Korenromp E, Low N, Unemo M, Abu-Raddad LJ, Chico RM, Smolak A, Newman L, Gottlieb S, Thwin SS, Broutet N, Taylor MM. 2019. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ 97:548–562P. doi:10.2471/BLT.18.228486. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

