Development of a Loop-Mediated Isothermal Amplification Method for Rapid and Visual Detection of Monkeypox Virus
- PMID: 36154444
- PMCID: PMC9603857
- DOI: 10.1128/spectrum.02714-22
Development of a Loop-Mediated Isothermal Amplification Method for Rapid and Visual Detection of Monkeypox Virus
Abstract
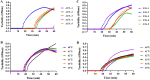
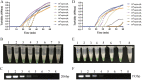
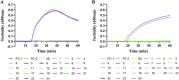
Monkeypox virus (MPXV) is a human pathogenic virus that belongs to the genus Orthopoxvirus. In 2022, MPXV caused an unprecedented number of infections in many countries. As it is difficult to distinguish MPXV from other pathogens by its symptoms in the early stage of infection, a rapid and reliable assay for MPXV detection is needed. In this study, we developed a loop-mediated isothermal amplification (LAMP) assay for the specific detection of MPXV and evaluated its application in simulated clinical samples. The A27L-1 and F3L-1 primer sets were identified as the optimal primers, and 63°C was the most appropriate reaction temperature for sequence amplification. The detection limits of the LAMP assay using primer sets A27L-1 and F3L-1 were both 20 copies/reaction mixture, which were >100-fold higher in terms of sensitivity, compared with conventional PCR. The LAMP assay findings were negative for all 21 non-MPXV pathogens, confirming the high specificity of our assay. All three types of simulated clinical samples were clearly identified by our LAMP assay, and the detection limits were consistent with the sensitivity results, indicating efficient clinical sample identification. Our rapid and reliable MPXV LAMP assay could be useful for MPXV detection and on-site diagnosis, especially in primary hospitals and rural areas. IMPORTANCE MPXV outbreaks rapidly grew in the first half of 2022, and this virus has been recognized as an increasing public health threat, particularly in the context of the COVID-19 pandemic. Thus, developing reliable and fast detection methods for MPXV is necessary.
Keywords: loop-mediated isothermal amplification; monkeypox virus; orthopoxvirus; rapid detection; visual detection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Loop-mediated isothermal amplification combined with lateral flow biosensor for rapid and sensitive detection of monkeypox virus.Front Public Health. 2023 Mar 24;11:1132896. doi: 10.3389/fpubh.2023.1132896. eCollection 2023. Front Public Health. 2023. PMID: 37033067 Free PMC article.
-
Loop-mediated isothermal amplification coupled with nanoparticle-based lateral flow biosensor for monkeypox virus detection.Talanta. 2024 Mar 1;269:125502. doi: 10.1016/j.talanta.2023.125502. Epub 2023 Dec 1. Talanta. 2024. PMID: 38070288
-
Loop-mediated isothermal amplification-based diagnostic assay for monkeypox virus infections.J Med Virol. 2009 Jun;81(6):1102-8. doi: 10.1002/jmv.21494. J Med Virol. 2009. PMID: 19382264
-
Monkeypox virus: a re-emergent threat to humans.Virol Sin. 2022 Aug;37(4):477-482. doi: 10.1016/j.virs.2022.07.006. Epub 2022 Jul 9. Virol Sin. 2022. PMID: 35820590 Free PMC article. Review.
-
Monkeypox virus diagnosis and laboratory testing.Rev Med Virol. 2023 Jan;33(1):e2404. doi: 10.1002/rmv.2404. Epub 2022 Nov 4. Rev Med Virol. 2023. PMID: 36331049 Review.
Cited by
-
Development of a Novel Loop-Mediated Isothermal Amplification Method for the Rapid Detection of Monkeypox Virus Infections.Viruses. 2022 Dec 28;15(1):84. doi: 10.3390/v15010084. Viruses. 2022. PMID: 36680124 Free PMC article.
-
Detection of the mpox virus using a robust recombinase-aided amplification-based approach.Biosaf Health. 2025 Mar 22;7(2):103-109. doi: 10.1016/j.bsheal.2025.03.001. eCollection 2025 Apr. Biosaf Health. 2025. PMID: 40453475 Free PMC article.
-
Mpox disease, diagnosis, and point of care platforms.Bioeng Transl Med. 2025 Jan 2;10(3):e10733. doi: 10.1002/btm2.10733. eCollection 2025 May. Bioeng Transl Med. 2025. PMID: 40385539 Free PMC article. Review.
-
Monkeypox Diagnosis in Clinical Settings: A Comprehensive Review of Best Laboratory Practices.Adv Exp Med Biol. 2024;1451:253-271. doi: 10.1007/978-3-031-57165-7_16. Adv Exp Med Biol. 2024. PMID: 38801583 Review.
-
Portable molecular diagnostic platform for rapid point-of-care detection of mpox and other diseases.Nat Commun. 2025 Mar 24;16(1):2875. doi: 10.1038/s41467-025-57647-3. Nat Commun. 2025. PMID: 40128193 Free PMC article.
References
-
- von Magnus P, Andersen EK, Petersen KB, Birch-Andersen A. 2009. A pox-like disease in cynomolgus monkeys. Acta Pathol Microbiol Scand 46:156–176. doi:10.1111/j.1699-0463.1959.tb00328.x. - DOI
-
- Likos AM, Sammons SA, Olson VA, Frace AM, Li Y, Olsen-Rasmussen M, Davidson W, Galloway R, Khristova ML, Reynolds MG, Zhao H, Carroll DS, Curns A, Formenty P, Esposito JJ, Regnery RL, Damon IK. 2005. A tale of two clades: monkeypox viruses. J Gen Virol 86:2661–2672. doi:10.1099/vir.0.81215-0. - DOI - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

