Factor-stimulated intrinsic termination: getting by with a little help from some friends
- PMID: 36154805
- PMCID: PMC9715273
- DOI: 10.1080/21541264.2022.2127602
Factor-stimulated intrinsic termination: getting by with a little help from some friends
Abstract
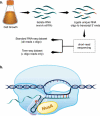
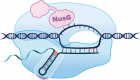
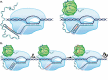
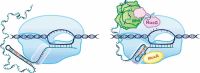
Transcription termination is known to occur via two mechanisms in bacteria, intrinsic termination (also frequently referred to as Rho-independent or factor-independent termination) and Rho-dependent termination. Based primarily on in vitro studies using Escherichia coli RNA polymerase, it was generally assumed that intrinsic termination and Rho-dependent termination are distinct mechanisms and that the signals required for intrinsic termination are present primarily within the nucleic acids. In this review, we detail recent findings from studies in Bacillus subtilis showing that intrinsic termination in this organism is highly stimulated by NusA, NusG, and even Rho. In NusA-stimulated intrinsic termination, NusA facilitates the formation of weak terminator hairpins and compensates for distal U-rich tract interruptions. In NusG-stimulated intrinsic termination, NusG stabilizes a sequence-dependent pause at the point of termination, which extends the time frame for RNA hairpins with weak terminal base pairs to form in either a NusA-stimulated or a NusA-independent fashion. In Rho-stimulated intrinsic termination, Rho prevents the formation of antiterminator-like RNA structures that could otherwise compete with the terminator hairpin. Combined, NusA, NusG, and Rho stimulate approximately 97% of all intrinsic terminators in B. subtilis. Thus, the general view that intrinsic termination is primarily a factor-independent process needs to be revised to account for recent findings. Moreover, the historical distinction between Rho-dependent and intrinsic termination is overly simplistic and needs to be modernized.
Keywords: Intrinsic termination; NusA; NusG; Rho; Rho-dependent termination.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Arndt KM, Chamberlin MJ.. RNA chain elongation by Escherichia coli RNA polymerase. Factors affecting the stability of elongating ternary complexes. J Mol Biol. 1990;213:79–108. - PubMed
-
- Korzheva N, Mustaev A, Kozlov M, et al. A structural model of transcription elongation. Science. 2000;289(5479):619–625. - PubMed
-
- Nudler E, Avetissova E, Markovtsov V, et al. Transcription processivity: protein-DNA interactions holding together the elongation complex. Science. 1996;273(5272):211–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
