Use of convalescent plasma in pregnant women with early stage COVID-19 infection in a tertiary care hospital in Dubai, February to March 2021: a case series study
- PMID: 36155102
- PMCID: PMC9509581
- DOI: 10.1186/s12884-022-05043-w
Use of convalescent plasma in pregnant women with early stage COVID-19 infection in a tertiary care hospital in Dubai, February to March 2021: a case series study
Abstract
Background: The use of COVID-19 convalescent plasma (CCP) for the treatment of SARS-CoV-2 infection in pregnancy is intriguing in view of its safety profile in pregnancy and historical precedence of the use of plasma for other viral illnesses. This study aimed to evaluate the use of CCP in pregnant women with early COVID-19 infection.
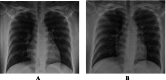
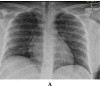
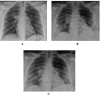
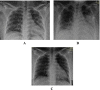
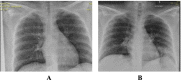
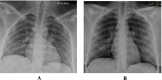
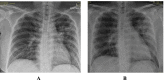
Methods: This is a retrospective case series study. We have included seven pregnant women admitted with early COVID-19 infection to a tertiary care hospital, Latifa Maternity Hospital in Dubai, United Arab Emirates between 12 February and 04 March 2021 and who consented to receive COVID-19 convalescent plasma as part of their treatment plan. Main outcomes measured were clinical and radiological features, laboratory tests, WHO clinical progression scale pre and post treatment, and maternal, fetal outcomes. COVID-19 clinical severity was classified according to the NIH guidelines for criteria of SARS-CoV-2. For the radiological features, a modified chest X-ray scoring system was used where each lung was divided into 6 zones (3 on each side upper, middle, and lower). Opacities were classified into reticular, ground glass, patchy and dense consolidations patterns.
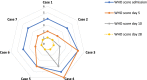
Results: Seven pregnant women with early COVID-19 were enrolled in this study, their mean age was 28 years (SD 3.6). Four had comorbidities: 2 with diabetes, 1 with asthma, and 1 was obese. Five patients were admitted with a WHO clinical progression score of 4 (hospitalized; with no oxygen therapy) and 2 with a score of 5 (hospitalized; oxygen by mask/nasal prongs). Upon follow up on day 10, 6 patients had a WHO score of 1 or 2 (asymptomatic/mild symptoms) indicating clinical recovery. Adverse reactions were reported in 2 patients, one reported a mild skin rash, and another developed transfusion related circulatory overload. All patients were discharged alive.
Conclusion: CCP seems to be a promising modality of treating COVID-19 infected pregnant women. However, further studies are needed to ascertain the efficacy of CCP in preventing progressive disease in the management of COVID-19 infection in pregnant women.
Keywords: COVID-19; Convalescent plasma; Dubai; Pregnancy; SARS-Cov-2.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
Similar articles
-
Reconvalescent plasma/camostat mesylate in early SARS-CoV-2 Q-PCR positive high-risk individuals (RES-Q-HR): a structured summary of a study protocol for a randomized controlled trial.Trials. 2021 May 17;22(1):343. doi: 10.1186/s13063-021-05181-0. Trials. 2021. PMID: 34001215 Free PMC article.
-
Randomized clinical trial to evaluate safety and efficacy of convalescent plasma use among hospitalized patients with COVID-19 (PERUCONPLASMA): a structured summary of a study protocol for a randomized controlled trial.Trials. 2021 May 17;22(1):342. doi: 10.1186/s13063-021-05189-6. Trials. 2021. PMID: 34001174 Free PMC article.
-
Covid-19 infection in pregnant women in Dubai: a case-control study.BMC Pregnancy Childbirth. 2021 Sep 28;21(1):658. doi: 10.1186/s12884-021-04130-8. BMC Pregnancy Childbirth. 2021. PMID: 34583679 Free PMC article.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review.Cochrane Database Syst Rev. 2020 May 14;5(5):CD013600. doi: 10.1002/14651858.CD013600. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Jul 10;7:CD013600. doi: 10.1002/14651858.CD013600.pub2. PMID: 32406927 Free PMC article. Updated.
-
Convalescent Plasma for Pregnant Women with COVID-19: A Systematic Literature Review.Viruses. 2021 Jun 22;13(7):1194. doi: 10.3390/v13071194. Viruses. 2021. PMID: 34206468 Free PMC article.
References
-
- World Health Organization Novel coronavirus (2019-nCoV) - situation report - 10 30. January 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, Debenham L, Llavall AC, Dixit A, Zhou D, Balaji R, Lee SI, Qiu X, Yuan M, Coomar D, Sheikh J, Lawson H, Ansari K, van Wely M, van Leeuwen E, Kostova E, Kunst H, Khalil A, Tiberi S, Brizuela V, Broutet N, Kara E, Kim CR, Thorson A, Oladapo OT, Mofenson L, Zamora J, Thangaratinam S, for PregCOV-19 Living Systematic Review Consortium Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

