Curcumin and capsaicin regulate apoptosis and alleviate intestinal inflammation induced by Clostridioides difficile in vitro
- PMID: 36155114
- PMCID: PMC9511736
- DOI: 10.1186/s12941-022-00533-3
Curcumin and capsaicin regulate apoptosis and alleviate intestinal inflammation induced by Clostridioides difficile in vitro
Abstract
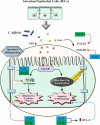
Background: The dramatic upsurge of Clostridioides difficile infection (CDI) by hypervirulent isolates along with the paucity of effective conventional treatment call for the development of new alternative medicines against CDI. The inhibitory effects of curcumin (CCM) and capsaicin (CAP) were investigated on the activity of toxigenic cell-free supernatants (Tox-S) of C. difficile RT 001, RT 126 and RT 084, and culture-filtrate of C. difficile ATCC 700057.
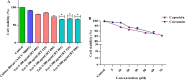
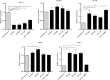
Methods: Cell viability of HT-29 cells exposed to varying concentrations of CCM, CAP, C. difficile Tox-S and culture-filtrate was assessed by MTT assay. Anti-inflammatory and anti-apoptotic effects of CCM and CAP were examined by treatment of HT-29 cells with C. difficile Tox-S and culture-filtrate. Expression of BCL-2, SMAD3, NF-κB, TGF-β and TNF-α genes in stimulated HT-29 cells was measured using RT-qPCR.
Results: C. difficile Tox-S significantly (P < 0.05) reduced the cell viability of HT-29 cells in comparison with untreated cells. Both CAP and CCM significantly (P < 0.05) downregulated the gene expression level of BCL-2, SMAD3, NF-κB and TNF-α in Tox-S treated HT-29 cells. Moreover, the gene expression of TGF-β decreased in Tox-S stimulated HT-29 cells by both CAP and CCM, although these reductions were not significantly different (P > 0.05).
Conclusion: The results of the present study highlighted that CCM and CAP can modulate the inflammatory response and apoptotic effects induced by Tox-S from different clinical C. difficile strains in vitro. Further studies are required to accurately explore the anti-toxin activity of natural components, and their probable adverse risks in clinical practice.
Keywords: Apoptosis; Capsaicin; Clostridioides difficile infection; Curcumin; Inflammation; Tox-S.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

