Safety and effectiveness of RBD-specific polyclonal equine F(ab´)2 fragments for the treatment of hospitalized patients with severe Covid-19 disease: A retrospective cohort study
- PMID: 36155545
- PMCID: PMC9512184
- DOI: 10.1371/journal.pone.0274796
Safety and effectiveness of RBD-specific polyclonal equine F(ab´)2 fragments for the treatment of hospitalized patients with severe Covid-19 disease: A retrospective cohort study
Abstract
Background: Passive immunotherapy has been evaluated as a therapeutic alternative for patients with COVID-19 disease. Equine polyclonal immunotherapy for COVID-19 (EPIC) showed adequate safety and potential efficacy in a clinical trial setting and obtained emergency use authorization in Argentina. We studied its utility in a real world setting with a larger population.
Methods: We conducted a retrospective cohort study at "Hospital de Campaña Escuela-Hogar" (HCEH) in Corrientes, Argentina, to assess safety and effectiveness of EPIC in hospitalized adults with severe COVID-19 pneumonia. Primary endpoints were 28-days all-cause mortality and safety. Mortality and improvement in modified WHO clinical scale at 14 and 21 days were secondary endpoints. Potential confounder adjustment was made by logistic regression weighted by the inverse of the probability of receiving the treatment (IPTW) and doubly robust approach.
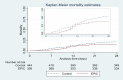
Findings: Subsequent clinical records of 446 non-exposed (Controls) and 395 exposed (EPIC) patients admitted between November 2020 and April 2021 were analyzed. Median age was 58 years and 56.8% were males. Mortality at 28 days was 15.7% (EPIC) vs. 21.5% (Control). After IPTW adjustment the OR was 0.66 (95% CI: 0.46-0.96) P = 0.03. The effect was more evident in the subgroup who received two EPIC doses (complete treatment, n = 379), OR 0.58 (95% CI 0.39 to 0.85) P = 0.005. Overall and serious adverse events were not significantly different between groups.
Conclusions: In this retrospective cohort study, EPIC showed adequate safety and effectiveness in the treatment of hospitalized patients with severe SARS-CoV-2 disease.
Conflict of interest statement
DHFS, FA, AAG, NAO, SNMP, LSN, FC, OIMI declare reimbursement for conduction of the observational study as investigators. EN, GL, WHB, DHG and LP report personal fees from Inmunova. SG and BK declare no competing interests. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous