Min pig skeletal muscle response to cold stress
- PMID: 36155652
- PMCID: PMC9512212
- DOI: 10.1371/journal.pone.0274184
Min pig skeletal muscle response to cold stress
Abstract
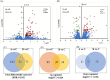
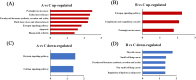
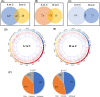
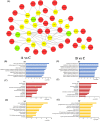
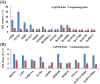
The increased sensitivity of pigs to ambient temperature is due to today's intensive farming. Frequent climate disasters increase the pressure on healthy pig farming. Min pigs are an indigenous pig breed in China with desirable cold resistance characteristics, and hence are ideal for obtaining cold-resistant pig breeds. Therefore, it is important to discover the molecular mechanisms that are activated in response to cold stress in the Min pig. Here, we conducted a transcriptomic analysis of the skeletal muscle of Min pigs under chronic low-temperature acclimation (group A) and acute short cold stress (group B). Cold exposure caused more genes to be upregulated. Totals of 125 and 96 differentially expressed genes (DEGs) were generated from groups A and B. Sixteen common upregulated DEGs were screened; these were concentrated in oxidative stress (SRXN1, MAFF), immune and inflammatory responses (ITPKC, AREG, MMP25, FOSL1), the nervous system (RETREG1, GADD45A, RCAN1), lipid metabolism (LRP11, LIPG, ITGA5, AMPD2), solute transport (SLC19A2, SLC28A1, SLCO4A1), and fertility (HBEGF). There were 102 and 73 genes that were specifically differentially expressed in groups A and B, respectively. The altered mRNAs were enriched in immune, endocrine, and cancer pathways. There were 186 and 91 differentially expressed lncRNAs generated from groups A and B. Analysis of the target genes suggested that they may be involved in regulating the MAPK signaling pathway for resistance to cold. The results of this study provide a comprehensive overview of cold exposure-induced transcriptional patterns in skeletal muscle of the Min pig. These results can guide future molecular studies of cold stress response in pigs for improving cold tolerance as a goal in breeding programs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

