Sex-specific effects of ethanol consumption in older Fischer 344 rats on microglial dynamics and Aβ(1-42) accumulation
- PMID: 36155778
- PMCID: PMC10251491
- DOI: 10.1016/j.alcohol.2022.08.013
Sex-specific effects of ethanol consumption in older Fischer 344 rats on microglial dynamics and Aβ(1-42) accumulation
Abstract
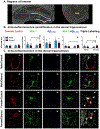
Chronic alcohol consumption, Alzheimer's disease (AD), and vascular dementia are all associated with cognitive decline later in life, raising questions about whether their underlying neuropathology may share some common features. Indeed, recent evidence suggests that ethanol exposure during adolescence or intermittent drinking in young adulthood increased neuropathological markers of AD, including both tau phosphorylation and beta-amyloid (Aβ) accumulation. The goal of the present study was to determine whether alcohol consumption later in life, a time when microglia and other neuroimmune processes tend to become overactive, would influence microglial clearance of Aβ(1-42), focusing specifically on microglia in close proximity to the neurovasculature. To do this, male and female Fischer 344 rats were exposed to a combination of voluntary and involuntary ethanol consumption from ∼10 months of age through ∼14 months of age. Immunofluorescence revealed profound sex differences in microglial co-localization, with Aβ(1-42) showing that aged female rats with a history of ethanol consumption had a higher number of iba1+ cells and marginally reduced expression of Aβ(1-42), suggesting greater phagocytic activity of Aβ(1-42) among females after chronic ethanol consumption later in life. Interestingly, these effects were most prominent in Iba1+ cells near neurovasculature that was stained with tomato lectin. In contrast, no significant effects of ethanol consumption were observed on any markers in males. These findings are among the first reports of a sex-specific increase in microglia-mediated phagocytosis of Aβ(1-42) by perivascular microglia in aged, ethanol-consuming rats, and may have important implications for understanding mechanisms of cognitive decline associated with chronic drinking.
Keywords: Aging; Amyloid beta; Chronic ethanol; Microglia; Sex differences.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Fibrillar Aβ triggers microglial proteome alterations and dysfunction in Alzheimer mouse models.Elife. 2020 Jun 8;9:e54083. doi: 10.7554/eLife.54083. Elife. 2020. PMID: 32510331 Free PMC article.
-
Early-life stress lastingly alters the neuroinflammatory response to amyloid pathology in an Alzheimer's disease mouse model.Brain Behav Immun. 2017 Jul;63:160-175. doi: 10.1016/j.bbi.2016.12.023. Epub 2016 Dec 25. Brain Behav Immun. 2017. PMID: 28027926
-
Tyrosine phosphorylation and palmitoylation of TRPV2 ion channel tune microglial beta-amyloid peptide phagocytosis.J Neuroinflammation. 2024 Sep 3;21(1):218. doi: 10.1186/s12974-024-03204-6. J Neuroinflammation. 2024. PMID: 39227967 Free PMC article.
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
-
[Pathological changes induced by amyloid-β in Alzheimer's disease].Yakugaku Zasshi. 2011 Jan;131(1):3-11. doi: 10.1248/yakushi.131.3. Yakugaku Zasshi. 2011. PMID: 21212607 Review. Japanese.
Cited by
-
Binge alcohol and the neuroendocrinology of the aging female.Front Neuroendocrinol. 2025 Jul;78:101201. doi: 10.1016/j.yfrne.2025.101201. Epub 2025 Jun 4. Front Neuroendocrinol. 2025. PMID: 40480422 Review.
-
NF-κB/NLRP3 Translational Inhibition by Nanoligomer Therapy Mitigates Ethanol and Advanced Age-Related Neuroinflammation.bioRxiv [Preprint]. 2024 Feb 28:2024.02.26.582114. doi: 10.1101/2024.02.26.582114. bioRxiv. 2024. Update in: J Leukoc Biol. 2025 Apr 23;117(4):qiaf024. doi: 10.1093/jleuko/qiaf024. PMID: 38464118 Free PMC article. Updated. Preprint.
-
Adolescent Alcohol and the Spectrum of Cognitive Dysfunction in Aging.Adv Exp Med Biol. 2025;1473:257-298. doi: 10.1007/978-3-031-81908-7_12. Adv Exp Med Biol. 2025. PMID: 40128483 Review.
-
Voluntary adolescent alcohol exposure does not robustly increase adulthood consumption of alcohol in multiple mouse and rat models.bioRxiv [Preprint]. 2024 Jul 21:2024.04.30.591674. doi: 10.1101/2024.04.30.591674. bioRxiv. 2024. Update in: Addict Neurosci. 2024 Sep;12:100171. doi: 10.1016/j.addicn.2024.100171. PMID: 38746266 Free PMC article. Updated. Preprint.
-
Intermittent Exposure to a Single Bottle of Ethanol Modulates Stress Sensitivity: Impact of Age at Exposure Initiation.Cells. 2023 Aug 3;12(15):1991. doi: 10.3390/cells12151991. Cells. 2023. PMID: 37566070 Free PMC article.
References
-
- Ahlner F, Sigström R, Rydberg Sterner T, Mellqvist Fässberg M, Kern S, Östling S, et al. (2018). Increased Alcohol Consumption Among Swedish 70-Year-Olds 1976 to 2016: Analysis of Data from The Gothenburg H70 Birth Cohort Studies, Sweden. Alcoholism: Clinical and Experimental Research, 42(12), 2403–2412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

