Association of Antenatal Steroid Exposure at 21 to 22 Weeks of Gestation With Neonatal Survival and Survival Without Morbidities
- PMID: 36156145
- PMCID: PMC9513645
- DOI: 10.1001/jamanetworkopen.2022.33331
Association of Antenatal Steroid Exposure at 21 to 22 Weeks of Gestation With Neonatal Survival and Survival Without Morbidities
Abstract
Importance: The provision of antenatal corticosteroids to pregnant patients at gestational age (GA) 22 6/7 weeks or less remains controversial and lacks support from randomized clinical trials.
Objective: To compare rates of survival and survival without major morbidities among infants born at GA 22 0/7 to 23 6/7 weeks after exposure to antenatal steroids at 22 6/7 weeks' gestation or less vs no exposure to antenatal steroids.
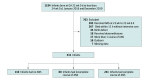
Design, setting, and participants: This cohort study enrolled infants born at GA 22 0/7 to 23 6/7 weeks between January 1, 2016, and December 31, 2019, at centers in the National Institute of Child Health and Human Development Neonatal Research Network. Infants who did not receive intensive care and infants with antenatal steroid exposure after GA 22 6/7 weeks were excluded.
Exposure: Infants were classified as having no, partial, or complete exposure to antenatal steroids.
Main outcomes and measures: The primary outcome was survival to discharge. The main secondary outcome was survival without major neonatal morbidity. The associations of differential exposures to antenatal steroids with outcomes were evaluated using logistic regression, adjusting for GA, sex, race, maternal education, small for GA status, mode of delivery, multiple birth, prolonged rupture of membranes, year of birth, and Neonatal Research Network center.
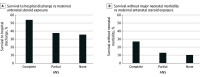
Results: A total of 431 infants (mean [SD] GA, 22.6 [0.5] weeks; 232 [53.8%] boys) were included, with 110 infants (25.5%) receiving no antenatal steroids, 80 infants (18.6%) receiving partial antenatal steroids, and 241 infants (55.9%) receiving complete antenatal steroids. Seventeen infants were exposed to antenatal steroids at GA 21 weeks. Among infants exposed to complete antenatal steroids, 130 (53.9%) survived to discharge, compared with 30 infants (37.5%) with partial antenatal steroid exposure and 239 infants (35.5%) with no antenatal steroids. Infants born after complete antenatal steroid exposure, compared with those without antenatal steroid exposure, were more likely to survive to discharge (adjusted odds ratio [aOR], 1.95 [95% CI, 1.07-3.56]) and to survive without major morbidity (aOR, 2.74 [95% CI, 1.19-6.30]).
Conclusions and relevance: In this retrospective cohort study, among infants born between GA 22 0/7 and 23 6/7 weeks who received intensive care, exposure to a complete course of antenatal steroids at GA 22 6/7 weeks or less was independently associated with greater odds of survival and survival without major morbidity. These data suggest that the use of antenatal steroids in patients at GA 22 6/7 weeks or less could be beneficial when active treatment is considered.
Conflict of interest statement
Figures
References
-
- Cahill AG, Kaimal AJ, Kuller JA, Turrentine MA; American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine . Use of antenatal corticosteroids at 22 weeks of gestation. Accessed August 24, 2022. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articl...