Radium-223 dichloride treatment in metastatic castration-resistant prostate cancer in Finland: A real-world evidence multicenter study
- PMID: 36156455
- PMCID: PMC9972699
- DOI: 10.1002/cam4.5262
Radium-223 dichloride treatment in metastatic castration-resistant prostate cancer in Finland: A real-world evidence multicenter study
Abstract
Background: Radium-233 dichloride is an alpha emitter that specifically targets bone metastases in prostate cancer. Results of a previously reported phase III randomized trial showed survival benefit for radium-223 compared to best supportive care in castration-resistant prostate cancer (CRPC) with bone metastases. However, real-world data are also needed with wider inclusion criteria.
Methods: We report results of a retrospective multicenter study including all patients with metastatic CRPC treated with radium-223 in all five university hospitals in Finland since the introduction of the treatment. We identified 160 patients who had received radium-223 in Finland in 2014-2019.
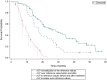
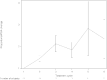
Results: The median overall survival (OS) was 13.8 months (range 0.5-57 months), and the median real-world progression-free survival (rwPFS) was 4.9 months (range 0.5-29.8 months). Alkaline phosphatase (ALP) values within the normal range before and during the radium-223 treatment or the reduction of elevated ALP to normal range during treatment were associated with better OS when compared to elevated ALP values before and during treatment (p < 0.0001). High prostate-specific antigen (PSA) level (≥100 μg/L) before radium-223 treatment was associated with poor OS compared to low PSA level (<20 μg/L) (p = 0.0001). Most patients (57%) experienced pain relief. Pain relief indicated better OS (p = 0.002). Radium-223 treatment was well tolerated. Toxicity was mostly low grade. Only 12.5% of the patients had grade III-IV adverse events, most commonly anemia, neutropenia, leucopenia, and thrombocytopenia.
Conclusion: Radium-223 was well tolerated in routine clinical practice, and most patients achieved pain relief. Pain relief, ALP normalization, lower baseline PSA, and PSA decrease during radium-223 treatment were prognostic for better survival. The efficacy of radium-223 in mCRPC as estimated using OS was comparable to earlier randomized trial in this retrospective real-world study. Our results support using radium-223 for mCRPC patients with symptomatic bone metastases even in the era of new-generation androgen receptor-targeted agents.
Keywords: bone metastases; castration-resistant prostate cancer; radium-233 (Ra-223) therapy; real-world evidence.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
AH, EL, PR, JS, and PA have nothing to disclose. OK had received conference participation, consultation, and lecture fees from Bayer, Ipsen, BMS, MSD Finland, Sanofi Genzyme, Lilly, Pierre Fabre, Roche, Merck, Jansen. TU had received conference participation, consultation and lecture fees from Amgen, Astellas, Bayer, BMS, Janssen, Orion, and Sanofi, and research funding from Bayer, Janssen, and Orion. MJ has been supported by Roche, Pierre Fabre, Amgen, MSD, and Abbvie for conference participation costs, and had received consulting or advisory honoraria from Amgen, Astra Zeneca, BMS, Ipsen, Merck, MSD, Novartis, Pfizer, Sanofi, and Sobi. HM has received research funding (not this study) from Finnish Cancer Foundations, Blue Earth Diagnostics, Merck, Philips, and Roche, and consultant fees from BMS, GSK, Jansen, MSD Finland, and Roche. KM had received consulting or advisory honoraria from Astellas, Bayer, Bristol‐Myers Squibb, Ipsen, Merck Sharp & Dohme, Merck–Pfizer alliance, Novartis, Roche, and Sanofi. MS has been supported by Pfizer, Novartis, BMS, Pierre Fabre, Roche, and Lilly for conference participation costs and received consultant fees from MSD, BMS, Roche, and Ipsen.
Figures




References
-
- Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. 2020;77(1):38‐52. - PubMed
-
- Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol 2000. –05;31(5):578–583. - PubMed
-
- Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(17):1653‐1654. - PubMed
-
- Parker C, Castro E, Fizazi K, et al. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2020;31(9):1119‐1134. - PubMed
-
- Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate Cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(5):479‐505. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

