The use of virtual visits for obesity pharmacotherapy in patients with overweight or obesity compared with in-person encounters
- PMID: 36156456
- PMCID: PMC9826334
- DOI: 10.1002/oby.23548
The use of virtual visits for obesity pharmacotherapy in patients with overweight or obesity compared with in-person encounters
Abstract
Objective: This study aimed to demonstrate noninferiority using telehealth in treating obesity with phentermine in patients with BMI ≥ 27 kg/m2 with comorbidities or BMI ≥ 30 compared with the standard in-person approach over a 90-day period.
Methods: A 12-week, randomized, prospective, single-center, open label trial compared the use of virtual visits versus in-person visits for the treatment of obesity using phentermine. The primary end point was percentage mean change in body weight from baseline to 12 weeks. A noninferiority approach assuming a 3% noninferiority region was used to assess effect size differences.
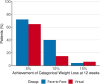
Results: The weight loss in the virtual visit arm was noninferior to the in-person arm at all time points. At 12 weeks, the mean change in weight was -6.5% among the virtual group and -7.7% among the in-person group. In addition, 65% of virtual patients and 71% of in-person patients demonstrated a weight reduction of at least 5%. There was no difference in medication tolerance, adherence, and compliance.
Conclusions: These results indicate that the virtual obesity pharmacotherapy visits in adults aged 18 to 65 years prescribed phentermine are effective and noninferior in achieving meaningful weight loss after 12 weeks. Future clinical trials are needed to better assess the effectiveness of televisits for obesity pharmacotherapy.
Trial registration: ClinicalTrials.gov NCT04614545.
© 2022 The Authors. Obesity published by Wiley Periodicals LLC on behalf of The Obesity Society.
Conflict of interest statement
W. Scott Butsch reports being a health care consultant for the Clinical Advisory Board for Rhythm Pharmaceuticals and the Obesity Educational Advisory Board for Novo Nordisk, A/S. Kevin M. Pantalone in the past 12 months reports receiving research support from Bayer, Novo Nordisk A/S, Merck, and Twinhealth, consulting honoraria from AstraZeneca, Bayer, Corcept Therapeutics, Diasome, Eli Lilly & Co., Merck, Novo Nordisk A/S, and Sanofi, and speaker honoraria from AstraZeneca, Corcept Therapeutics, Merck, and Novo Nordisk A/S. The other authors declared no conflict of interest.
Figures



References
-
- Jensen MD, Ryan DH, Apovian CM, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation. 2014;129(25 suppl 2):S102‐S140. - PMC - PubMed
-
- Bersoux S, Byun TH, Chaliki SS, Poole KG. Pharmacotherapy for obesity: what you need to know. Cleve Clin J Med. 2017;84:951‐958. - PubMed
-
- Mann T, Tomiyama AJ, Westling E, Lew AM, Samuels B, Chatman J. Medicare's search for effective obesity treatments: diets are not the answer. Am Psychol. 2007;62:220‐233. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

