[Determination of the species origin and thrombin-like enzyme content of Bothrops atrox venom by ultra-high performance liquid chromatography-tandem mass spectrometry based on marker peptide]
- PMID: 36156627
- PMCID: PMC9520370
- DOI: 10.3724/SP.J.1123.2021.12020
[Determination of the species origin and thrombin-like enzyme content of Bothrops atrox venom by ultra-high performance liquid chromatography-tandem mass spectrometry based on marker peptide]
Abstract
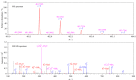
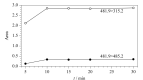
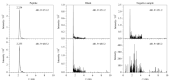
Snake venom thrombin drugs are hemostatic drugs prepared from Agkistrodon halys venom, and the main active ingredients are snake venom thrombin-like enzymes (svTLEs). The svTLEs derived from different snake species differ in their structures, hemostatic mechanisms, and pharmacological effects. Therefore, accurate identification of the source of snake venom species and determination of the svTLE content are essential to ensure the quality of these products. Based on proteomics technology, the marker peptides of svTLEs from Bothrops atrox were screened with species specificity for the first time in this study, and an ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) method for species identification and determination of the svTLE content of Bothrops atrox was established. After reductive alkylation and trypsin enzymolysis of the purified svTLE from Bothrops atrox, enzymatic peptide fragments were obtained and determined by easy-nano liquid chromatography-quadrupole/electrostatic field orbitrap high resolution mass spectrometry (Nano LC-Q-Exactive-MS). The mass spectrum data were analyzed by Proteome Discoverer 2.2 software. The maker peptide "EAYNGLPAK", which characterized the svTLE from Bothrops atrox, was finally screened and validated by comparison of the basic local alignment search tool (BLAST) with the NCBI and UniProt databases. For the marker peptide, the enzymolysis temperature, enzymolysis time and amount of enzyme for the sample preparation were optimized. The optimized enzymolysis conditions were as follows: enzymolysis temperature, 37 ℃; enzymolysis time, 4 h; and amount of enzyme, 10 μL. A qualitative and quantitative detection method based on UHPLC-MS/MS was established by optimizing the chromatographic and mass spectrometric conditions. Accordingly, 20 mg of the evenly mixed sample was weighed and placed in a 100 mL volumetric flask. Then, 25 mmol/L ammonium bicarbonate solution was added to dissolve the sample, and the solution was diluted to the scale. Precisely 1.00 mL of the solution was extracted; subsequently, addition of 10 μL trypsin solution was added, followed by shaking, and the mixture was placed in an incubator for 4 h to induce enzymolysis at a constant temperature of 37 ℃. The mixture was subsequently removed from the incubator, cooled to ambient temperature, centrifuged at 12000 r/min for 10 min, and analyzed by LC-MS. Separation was performed on the UPLC system with a Thermo Hypersil GOLD C18 column (100 mm×2.1 mm, 3.0 μm) under the gradient elution of acetonitrile containing 0.1% (v/v) acetic acid and water containing 0.1%(v/v) acetic acid, at a flow rate of 0.3 mL/min, column temperature of 30 ℃, and injection volume of 2 μL. The maker peptides were determined in the electrospray positive ionization (ESI+) and multiple reaction monitoring (MRM) modes using the external standard curve method. The detection ions were m/z 481.9> 315.2 and 481.9> 485.2. There was a good linear relationship between the mass concentration of the marker peptide and the chromatographic peak area in the range of 2.5-30 ng/mL, and the correlation coefficient (r) was 0.9996, The limit of detection (S/N=3) and limit of quantification (S/N=10) were 2.5 mg/kg and 6.25 mg/kg, respectively. At spiked levels of 40, 80, and 120 mg/kg, the recoveries of the marker peptides were 95.5%-101.9%, while the relative standard deviations (RSDs) of the results for parallel analyses at various spiked levels were 1.1%-3.2%. The developed method is simple, rapid, sensitive, and specific, and it can be used for the identification of Bothrops atrox venom species and determination of the svTLE content. The findings of this study would help ensure the quality of hemocoagulase products from the relevant source and provide a reference for the quality control of other snake venom products.
蛇毒血凝酶类药物是以蝮蛇蛇毒为原料制备的止血药,主要活性成分为蛇毒类凝血酶(svTLEs)。不同蛇种来源的svTLEs结构不同,止血机制不同,药理作用也存在差异,因此准确鉴别蛇毒种属来源和svTLEs含量对于保障该类产品的质量至关重要。研究基于蛋白质组学技术,筛选出了具有种属特异性的矛头蝮蛇svTLE特征肽,并建立了基于特征肽的超高效液相色谱-串联质谱(UHPLC-MS/MS)检测矛头蝮蛇蛇毒种属来源及类凝血酶含量的方法。采用胰蛋白酶对纯化的矛头蝮蛇svTLE进行酶解,利用纳升液相色谱-四极杆/静电场轨道阱高分辨质谱(Nano LC-Q-Exactive-MS)和Proteome Discoverer 2.2软件分别进行多肽的检测和鉴定,通过BLAST搜索与Uniprot数据库对比分析,筛选出具有种属特异性的矛头蝮蛇svTLEs特征肽“EAYNGLPAK”。针对该特征肽对酶解温度、酶解时间和酶用量等样品前处理方法进行了优化,利用超高效液相色谱-串联质谱,以m/z 481.9>315.2和481.9>485.2作为检测离子对,采用ESI+模式进行了多反应监测(MRM)定性定量分析。结果显示,特征肽在2.5~30 ng/mL范围内线性关系良好,相关系数(r)大于0.9996,多水平加标回收率范围为95.5%~101.9%,各水平平行测定结果的相对标准偏差(RSD)为1.1%~3.2%,完全能够满足实际样品检测需求。方法简便快捷,灵敏度高,专属性强,可用于矛头蝮蛇蛇毒种属鉴别及svTLE含量测定,从源头保证血凝酶类产品的质量,并可为其他蛇毒类产品的质量控制提供参考。
Keywords:
Figures
Similar articles
-
[Analysis and identification of suspected snake venom samples using nano-ultra-high performance liquid chromatography-high resolution mass spectrometry].Se Pu. 2023 Feb;41(2):122-130. doi: 10.3724/SP.J.1123.2022.08009. Se Pu. 2023. PMID: 36725708 Free PMC article. Chinese.
-
[Determination of donkey skin ingredients in Asini Corii Colla by ultra-high performance liquid chromatography-tandem mass spectrometry].Se Pu. 2021 Nov;39(11):1255-1260. doi: 10.3724/SP.J.1123.2021.02003. Se Pu. 2021. PMID: 34677021 Free PMC article. Chinese.
-
[Simultaneous determination of beauvericin and four enniatins in eggs by ultra-performance liquid chromatography-tandem mass spectrometry coupled with cold-induced liquid-liquid extraction and dispersive solid phase extraction].Se Pu. 2021 Dec;39(12):1331-1339. doi: 10.3724/SP.J.1123.2021.02015. Se Pu. 2021. PMID: 34812005 Free PMC article. Chinese.
-
The Current State-of-the-Art Identification of Unknown Proteins Using Mass Spectrometry Exemplified on De Novo Sequencing of a Venom Protease from Bothrops moojeni.Molecules. 2022 Aug 5;27(15):4976. doi: 10.3390/molecules27154976. Molecules. 2022. PMID: 35956926 Free PMC article. Review.
-
[Advances in high-throughput proteomic analysis].Se Pu. 2021 Feb;39(2):112-117. doi: 10.3724/SP.J.1123.2020.08023. Se Pu. 2021. PMID: 34227342 Free PMC article. Review. Chinese.
Cited by
-
[Analysis and identification of suspected snake venom samples using nano-ultra-high performance liquid chromatography-high resolution mass spectrometry].Se Pu. 2023 Feb;41(2):122-130. doi: 10.3724/SP.J.1123.2022.08009. Se Pu. 2023. PMID: 36725708 Free PMC article. Chinese.
References
-
- Liu Y P, Kong Y, Li Q. Pharmaceutical Biotechnology, 2017, 24(4): 353
- 刘艳坡, 孔毅, 李谦. 药物生物技术, 2017, 24(4): 353
-
- Cao D. Chinese Journal of Drug Evaluation, 2016, 33(4): 216
- 曹迪. 中国药物评价, 2016, 33(4): 216
-
- Guan L F, Chi C W, Yuan M. Thromb Res, 1984, 35(3): 301 - PubMed
-
- Shen W Y, Wang H Y, Xue Y, et al.. Journal of Shenyang Pharmaceutical University, 2010, 27(7): 583
- 沈文彧, 王宏英, 薛雁, et al.. 沈阳药科大学学报, 2010, 27 (7): 583
-
- Li X N, Sun D, Li X L, et al.. Journal of Snake, 2016, 28(2): 117
- 李秀娜, 孙东, 李秀琳, et al.. 蛇志, 2016, 28(2): 117
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials