Remdesivir: the first FDA-approved anti-COVID-19 Treatment for Young Children
- PMID: 36156901
- PMCID: PMC9491826
- DOI: 10.15190/d.2022.10
Remdesivir: the first FDA-approved anti-COVID-19 Treatment for Young Children
Abstract
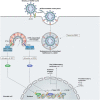
Following the emergence of the SARS-CoV-2 pandemic, finding efficient forms of treatment is seen as a priority for both adults and children. On April 25, 2022, remdesivir has become the first United States Food and Drug Administration (FDA) approved COVID-19 treatment for young children, specifically ≥28-days-old children, weighing ≥3 kilograms, who are either hospitalized or non-hospitalized, showing a high risk for progression to severe COVID-19 (prone to hospitalization or death). This new approval, which expands its already FDA-approved use in adults to young children, is supported by the CARAVAN study (a phase 2/3 single-arm, open-label study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of remdesivir (GS-5734™) in participants, from birth to < 18 years of age, with COVID-19). This study is in progress, with an estimated primary completion in February 2023. While positive effects of remdesivir have been ascertained through various studies, controversy has surrounded remdesivir since its initial FDA approval in 2020 due to the contradictory results obtained by various studies. However, many case reports state its positive effects on the outcome of the patients, encouraging an optimistic vision for the future.
Keywords: COVID-19; benefits and limitations.; pediatric; remdesivir; young children.
Copyright © 2022, Chera A et al., Applied Systems and Discoveries Journals.
Conflict of interest statement
Conflict of interests: The authors declare no conflicts of interest.
Figures
Similar articles
-
Remdesivir Efficacy and Tolerability in Children with COVID-19-Associated Allergic Comorbidities such as Asthma, Allergic Rhinitis, and Atopic Dermatitis.Children (Basel). 2023 Apr 29;10(5):810. doi: 10.3390/children10050810. Children (Basel). 2023. PMID: 37238359 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Remdesivir for COVID-19 in Hospitalized Children: A Phase 2/3 Study.Pediatrics. 2024 Mar 1;153(3):e2023063775. doi: 10.1542/peds.2023-063775. Pediatrics. 2024. PMID: 38332740 Clinical Trial.
-
Remdesivir for the treatment of COVID 19: review of the pharmacological properties, safety and clinical effectiveness.Expert Opin Drug Saf. 2021 Nov;20(11):1299-1307. doi: 10.1080/14740338.2021.1962284. Epub 2021 Aug 16. Expert Opin Drug Saf. 2021. PMID: 34338121 Review.
-
Remdesivir against COVID-19 and Other Viral Diseases.Clin Microbiol Rev. 2020 Oct 14;34(1):e00162-20. doi: 10.1128/CMR.00162-20. Print 2020 Dec 16. Clin Microbiol Rev. 2020. PMID: 33055231 Free PMC article. Review.
Cited by
-
Advances and Challenges in Antiviral Development for Respiratory Viruses.Pathogens. 2024 Dec 31;14(1):20. doi: 10.3390/pathogens14010020. Pathogens. 2024. PMID: 39860981 Free PMC article. Review.
-
Decoy peptides effectively inhibit the binding of SARS-CoV-2 to ACE2 on oral epithelial cells.Heliyon. 2023 Nov 20;9(12):e22614. doi: 10.1016/j.heliyon.2023.e22614. eCollection 2023 Dec. Heliyon. 2023. PMID: 38107325 Free PMC article.
-
An Updated Systematic Review on Remdesivir's Safety and Efficacy in Patients Afflicted With COVID-19.Cureus. 2023 Aug 7;15(8):e43060. doi: 10.7759/cureus.43060. eCollection 2023 Aug. Cureus. 2023. PMID: 37680394 Free PMC article. Review.
-
Mechanistic Understanding of Dexamethasone-Mediated Protection against Remdesivir-Induced Hepatotoxicity.Mol Pharmacol. 2024 Jun 18;106(1):71-82. doi: 10.1124/molpharm.124.000894. Mol Pharmacol. 2024. PMID: 38769019 Free PMC article.
-
Discovery of Pyrano[2,3-c]pyrazole Derivatives as Novel Potential Human Coronavirus Inhibitors: Design, Synthesis, In Silico, In Vitro, and ADME Studies.Pharmaceuticals (Basel). 2024 Feb 2;17(2):198. doi: 10.3390/ph17020198. Pharmaceuticals (Basel). 2024. PMID: 38399412 Free PMC article.
References
-
- Remdesivir for the Treatment of Covid-19 - Final Report. Beigel John H, Tomashek Kay M, Dodd Lori E, Mehta Aneesh K, Zingman Barry S, Kalil Andre C, Hohmann Elizabeth, Chu Helen Y, Luetkemeyer Annie, Kline Susan, Lopez de Castilla Diego, Finberg Robert W, Dierberg Kerry, Tapson Victor, Hsieh Lanny, Patterson Thomas F, Paredes Roger, Sweeney Daniel A, Short William R, Touloumi Giota, Lye David Chien, Ohmagari Norio, Oh Myoung-Don, Ruiz-Palacios Guillermo M, Benfield Thomas, Fätkenheuer Gerd, Kortepeter Mark G, Atmar Robert L, Creech C Buddy, Lundgren Jens, Babiker Abdel G, Pett Sarah, Neaton James D, Burgess Timothy H, Bonnett Tyler, Green Michelle, Makowski Mat, Osinusi Anu, Nayak Seema, Lane H Clifford. The New England journal of medicine. 2020;383(19):1813–1826. - PMC - PubMed
-
- Characteristics and conflicting recommendations of clinical practice guidelines for COVID-19 management in children: A scoping review. Quincho-Lopez Alvaro, Chávez-Rimache Lesly, Montes-Alvis José, Taype-Rondan Alvaro, Alvarado-Gamarra Giancarlo. Travel medicine and infectious disease. 2022;48:102354. - PMC - PubMed
-
- Therapeutic Strategies for COVID-19 Lung Disease in Children. Gatti Elisabetta, Piotto Marta, Lelii Mara, Pensabene Mariacarola, Madini Barbara, Cerrato Lucia, Hassan Vittoria, Aliberti Stefano, Bosis Samantha, Marchisio Paola, Patria Maria Francesca. Frontiers in pediatrics. 2022;10:829521. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
