The Role of C-Reactive Protein as a Prognostic Biomarker in Patients with Early Breast Cancer Treated with Neoadjuvant Chemotherapy
- PMID: 36156910
- PMCID: PMC9453660
- DOI: 10.1159/000522606
The Role of C-Reactive Protein as a Prognostic Biomarker in Patients with Early Breast Cancer Treated with Neoadjuvant Chemotherapy
Abstract
Background: C-reactive protein (CRP) is an acute phase reactant influenced by inflammation and tissue damage. Elevated CRP levels have been associated with poor outcome of various cancers including breast cancer. However, evidence regarding a potential impact of CRP levels on outcome of neoadjuvant chemotherapy (NACT) in patients with early breast cancer (EBC) is insufficient.
Methods: Patients who had received NACT for EBC and had available data regarding CRP levels before therapy, pathologic complete remission (pCR), and follow-up were included. The association between CRP at baseline and outcome parameters was analyzed.
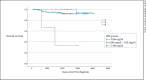
Results: 152 women were included in this analysis; median follow-up was 5.8 years. No association between CRP at baseline and pCR rates could be detected. 6.6% of the patients developed a local recurrence, 10.5% developed a distant recurrence, and 5.2% died from breast cancer. A negative correlation (Spearman-Rho) between CRP at baseline and overall survival (OS) (correlation coefficient (CC) -0.255; p = 0.45), disease-free survival (DFS) (CC -0.348; p = 0.075), local recurrence-free survival (LRFS) (CC -0.245; p = 0.327), and distant DFS (DDFS) (CC -0.422; p = 0.057) was not statistically significant, although especially in DFS and DDFS a strong trend was detected. The probability of death from breast cancer was 2% if the CRP was <0.08 mg/dL and 40% if the CRP was >2.08 mg/dL; this association was highly statistically significant (χ2; p < 0.001). These results were independent from age, estrogen and progesterone receptor status, HER2 status, nodal status, and grading. The hazard ratio for OS was 5.75 (p = 0.004) for CRP <0.08 mg/dL versus CRP >2.08 mg/dL.
Discussion/conclusion: CRP at baseline is not predictive for pCR in EBC after NACT in our patient dataset. However, an association of parameters of long-term prognosis with CRP could be demonstrated. Although the correlations of higher CRP levels at baseline and shorter OS, DFS, LRFS, and DDFS were not significant, a strong trend could be detected that was reproduced in the analysis of different groups of CRP levels and the probability of breast cancer mortality. Higher CRP levels are indicating a worse prognosis in EBC after NACT in this retrospective analysis. These results justify further investigation of CRP not as a predictive parameter for pCR but as a biomarker of long-term prognosis in EBC in prospective trials and may lead to therapeutic approaches with the aim of lowering CRP levels.
Keywords: Biomarker; Breast cancer; C-reactive protein; Early disease; Neoadjuvant therapy.
Copyright © 2022 by S. Karger AG, Basel.
Conflict of interest statement
H.C.K. received honoraria and travel support from AstraZeneca, Pfizer, Roche, Daiichi Sankyo, Tesaro, MSD, onkowissen, Eli Lilly, SurgVision, Exact Sciences, und Genomic Health and holds stock of Theraclion und Phaon scientific. C.K.L. received honoraria from Roche, Novartis, Pfizer, Amgen, Astra Zeneca, onkowissen, Gilead Sciences, SeaGen, Sanofi-Genzyme, and MSD and research funding from Gilead Sciences. A.K.B. received honoraria and travel support from Amgen, AstraZeneca, Daiichi Sankyo, Hexal, Novartis, Pfizer, Roche, MSD, and SeaGen. O.H. received honoraria and travel support from Amgen, AstraZeneca, Daiichi Sankyo, Hexal, Novartis, Pfizer, Roche, MSD, Riemser, SeaGen, Gilead, and Eisai. A.E., S.W., M.S., and M.S. have nothing to disclose.
Similar articles
-
Outcome after neoadjuvant chemotherapy in estrogen receptor-positive and progesterone receptor-negative breast cancer patients: a pooled analysis of individual patient data from ten prospectively randomized controlled neoadjuvant trials.Breast Cancer Res Treat. 2018 Jan;167(1):59-71. doi: 10.1007/s10549-017-4480-5. Epub 2017 Sep 5. Breast Cancer Res Treat. 2018. PMID: 28875243
-
Phase III study of long-term prognosis of estrogen receptor-positive early breast cancer treated with neoadjuvant endocrine therapy with/without adjuvant chemotherapy.Breast Cancer Res Treat. 2023 Jun;199(2):231-241. doi: 10.1007/s10549-023-06874-7. Epub 2023 Mar 22. Breast Cancer Res Treat. 2023. PMID: 36947277 Free PMC article. Clinical Trial.
-
Validation of Residual Proliferative Cancer Burden as a Predictor of Long-Term Outcome Following Neoadjuvant Chemotherapy in Patients with Hormone Receptor-Positive/Human Epidermal Growth Receptor 2-Negative Breast Cancer.Oncologist. 2020 Sep;25(9):e1355-e1362. doi: 10.1634/theoncologist.2020-0201. Epub 2020 Jul 21. Oncologist. 2020. PMID: 32618068 Free PMC article.
-
Overview of resistance to systemic therapy in patients with breast cancer.Adv Exp Med Biol. 2007;608:1-22. doi: 10.1007/978-0-387-74039-3_1. Adv Exp Med Biol. 2007. PMID: 17993229 Review.
-
C-Reactive Protein and Breast Cancer: New Insights from Old Molecule.Int J Breast Cancer. 2015;2015:145647. doi: 10.1155/2015/145647. Epub 2015 Nov 26. Int J Breast Cancer. 2015. PMID: 26693355 Free PMC article. Review.
Cited by
-
A Visual Distance-Based Capillary Immunoassay Using Biomimetic Polymer Nanoparticles for Highly Sensitive and Specific C-Reactive Protein Quantification.Int J Mol Sci. 2024 Sep 10;25(18):9771. doi: 10.3390/ijms25189771. Int J Mol Sci. 2024. PMID: 39337259 Free PMC article.
-
Low-Invasive Biomarkers of Canine Mammary Tumours.Vet Med Sci. 2025 Mar;11(2):e70280. doi: 10.1002/vms3.70280. Vet Med Sci. 2025. PMID: 40095734 Free PMC article. Review.
-
High C-reactive protein is associated with the efficacy of neoadjuvant chemotherapy for hormone receptor-positive breast cancer.Medicine (Baltimore). 2024 Nov 29;103(48):e40775. doi: 10.1097/MD.0000000000040775. Medicine (Baltimore). 2024. PMID: 39612416 Free PMC article.
References
-
- Bode JG, Albrecht U, Häussinger D, Heinrich PC, Schaper F. Hepatic acute phase proteins: regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-κB-dependent signaling. Eur J Cell Biol. 2012;91:496–505. - PubMed
-
- Du Clos TW. Function of C-reactive protein. Ann Med. 2000;32((32)):274–8. - PubMed
-
- Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol. 2001;38:189–97. - PubMed
-
- Pepys MB. C-reactive protein fifty years on. Lancet. 1981;1:653–7. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous