Effectiveness, safety, and drug sustainability of biologics in elderly patients with inflammatory bowel disease: A retrospective study
- PMID: 36156919
- PMCID: PMC9476849
- DOI: 10.3748/wjg.v28.i33.4823
Effectiveness, safety, and drug sustainability of biologics in elderly patients with inflammatory bowel disease: A retrospective study
Abstract
Background: Biologic therapy resulted in a significant positive impact on the management of inflammatory bowel disease (IBD) however data on the efficacy and side effects of these therapies in the elderly is scant.
Aim: To evaluate retrospectively the drug sustainability, effectiveness, and safety of the biologic therapies in the elderly IBD population.
Methods: Consecutive elderly (≥ 60 years old) IBD patients, treated with biologics [infliximab (IFX), adalimumab (ADAL), vedolizumab (VDZ), ustekinumab (UST)] followed at the McGill University Inflammatory Bowel Diseases Center were included between January 2000 and 2020. Efficacy was measured by clinical scores at 3, 6-9 and 12-18 mo after initiation of the biologic therapy. Patients completing induction therapy were included. Adverse events (AEs) or serious AE were collected during and within three months of stopping of the biologic therapy.
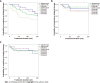
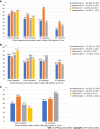
Results: We identified a total of 147 elderly patients with IBD treated with biologicals during the study period, including 109 with Crohn's disease and 38 with ulcerative colitis. Patients received the following biologicals: IFX (28.5%), ADAL (38.7%), VDZ (15.6%), UST (17%). The mean duration of biologic treatment was 157.5 (SD = 148) wk. Parallel steroid therapy was given in 34% at baseline, 19% at 3 mo, 16.3% at 6-9 mo and 6.5% at 12-18 mo. The remission rates at 3, 6-9 and 12-18 mo were not significantly different among biological therapies. Kaplan-Meyer analysis did not show statistical difference for drug sustainability (P = 0.195), time to adverse event (P = 0.158) or infection rates (P = 0.973) between the four biologics studied. The most common AEs that led to drug discontinuation were loss of response, infusion/injection reaction and infection.
Conclusion: Current biologics were not different regarding drug sustainability, effectiveness, and safety in the elderly IBD population. Therefore, we are not able to suggest a preferred sequencing order among biologicals.
Keywords: Adverse events; Biologics; Efficacy; Elderly; Inflammatory bowel disease; Safety.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: Hahn GD, Qatomah A, Wang A, Boodaghians L, Liu Chen Kiow J, Al Ali M, and Wild G declared no conflict of interest; LeBlanc JF has been a speaker or advisory board member for Janssen and Takeda; Golovics PA has been a speaker for AbbVie, Takeda, Fresenius, Ferring; Wetwittayakhlang P has been a speaker and/or advisory board member: Takeda, Pfizer, Janssen, Ferring, A. Menerini, and MSD; Afif W has been a speaker for Janssen, Prometheus, Dynacare, Takeda, AbbVie Theradiag; Bitton A has been a member of Advisory Boards - Abbvie, Pfizer, Takeda, Janssen, Merck; Speaker’s bureau - Abbvie, Janssen, Takeda, Pfizer; Lakatos PL has been a speaker and/or advisory board member for AbbVie, Arena, Falk Pharma GmbH, Ferring, Genetech, Janssen, Kyowa Hakko Kirin Pharma, Mitsubishi Tanabe Pharma Corporation, MSD, Pfizer, Roche, Shire, Takeda and Tillots, and has received unrestricted research grants from AbbVie, MSD and Pfizer; Bessissow T has been a speaker or advisory board member for Takeda, Janssen, Abbvie, Merck, Pfizer, Pendopharm, Ferring, Shire, Sandoz, BMS, Roche, Fresenius Kabi, Viatris.
Figures
Similar articles
-
Evaluation of adverse clinical outcomes in patients with inflammatory bowel disease receiving different sequences of first- and second-line biologic treatments: findings from ROTARY.BMC Gastroenterol. 2024 Sep 17;24(1):314. doi: 10.1186/s12876-024-03378-6. BMC Gastroenterol. 2024. PMID: 39289603 Free PMC article.
-
Real-World Persistence of Successive Biologics in Patients With Inflammatory Bowel Disease: Findings From ROTARY.Inflamm Bowel Dis. 2024 Oct 3;30(10):1776-1787. doi: 10.1093/ibd/izad245. Inflamm Bowel Dis. 2024. PMID: 37921344 Free PMC article.
-
Combination biologic therapy in pediatric inflammatory bowel disease: Safety and efficacy over a minimum 12-month follow-up period.J Pediatr Gastroenterol Nutr. 2024 Jul;79(1):54-61. doi: 10.1002/jpn3.12179. Epub 2024 Mar 13. J Pediatr Gastroenterol Nutr. 2024. PMID: 38477410
-
Selection strategy of second-line biologic therapies in adult patients with ulcerative colitis following prior biologic treatment failure: Systematic review and meta-analysis.Pharmacol Res. 2024 Apr;202:107108. doi: 10.1016/j.phrs.2024.107108. Epub 2024 Feb 24. Pharmacol Res. 2024. PMID: 38403257
-
Comparative efficacy and safety of infliximab and vedolizumab therapy in patients with inflammatory bowel disease: a systematic review and meta-analysis.BMC Gastroenterol. 2022 Jun 8;22(1):291. doi: 10.1186/s12876-022-02347-1. BMC Gastroenterol. 2022. PMID: 35676620 Free PMC article.
Cited by
-
Inflammatory Bowel Diseases in the Elderly: A Focus on Disease Characteristics and Biological Therapy Patterns.J Clin Med. 2024 May 8;13(10):2767. doi: 10.3390/jcm13102767. J Clin Med. 2024. PMID: 38792308 Free PMC article.
-
Efficacy and Safety of Biological Therapies and JAK Inhibitors in Older Patients with Inflammatory Bowel Disease.Cells. 2023 Jun 26;12(13):1722. doi: 10.3390/cells12131722. Cells. 2023. PMID: 37443755 Free PMC article. Review.
-
Frequency of Biological Drug Use in Older Patients with Immune-Mediated Inflammatory Diseases: Results from the Large-Scale Italian VALORE Distributed Database Network.BioDrugs. 2025 May;39(3):499-512. doi: 10.1007/s40259-025-00716-2. Epub 2025 Apr 3. BioDrugs. 2025. PMID: 40180772 Free PMC article.
References
-
- Jeuring SF, van den Heuvel TR, Zeegers MP, Hameeteman WH, Romberg-Camps MJ, Oostenbrug LE, Masclee AA, Jonkers DM, Pierik MJ. Epidemiology and Long-term Outcome of Inflammatory Bowel Disease Diagnosed at Elderly Age-An Increasing Distinct Entity? Inflamm Bowel Dis. 2016;22:1425–1434. - PubMed
-
- Gisbert JP, Chaparro M. Systematic review with meta-analysis: inflammatory bowel disease in the elderly. Aliment Pharmacol Ther. 2014;39:459–477. - PubMed
-
- Hruz P, Juillerat P, Kullak-Ublick GA, Schoepfer AM, Mantzaris GJ, Rogler G on behalf of Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology. Management of the Elderly Inflammatory Bowel Disease Patient. Digestion. 2020;101 Suppl 1:105–119. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

