Probing the roles of pH and ionic strength on electrostatic binding of tetracycline by dissolved organic matters: Reevaluation of modified fitting model
- PMID: 36156988
- PMCID: PMC9488040
- DOI: 10.1016/j.ese.2021.100133
Probing the roles of pH and ionic strength on electrostatic binding of tetracycline by dissolved organic matters: Reevaluation of modified fitting model
Abstract
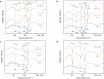
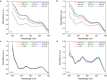
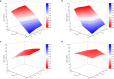
The binding performance of dissolved organic matters (DOM) plays a critical role in the migration, diffusion and removal of various residual pollutants in the natural water environment. In the current study, four typical DOMs (including bovine serum proteins BSA (proteins), sodium alginate SAA (polysaccharides), humic acid HA and fulvic acid FA (humus)) are selected to investigate the binding roles in zwitterionic tetracycline (TET) antibiotic under various ionic strength (IS = 0.001-0.1 M) and pH (5.0-9.0). The dialysis equilibration technique was employed to determine the binding concentrations of TET, and the influence of IS and pH on binding performance was evaluated via UV-vis spectroscopy, total organic carbon (TOC), and Excitation-Emission-Matrix spectra (EEM), zeta potentials and molecule size distribution analysis. Our results suggested that carboxyl and phenolic hydroxyl were identified as the main contributors to TET binding based on the fourier transform infrared spectroscopy (FTIR) analysis, and the binding capability of four DOMs followed as HA > FA » BSA > SAA. The biggest binding concentrations of TET by 10 mg C/L HA, FA, BSA and SAA were 0.863 μM, 0.487 μM, 0.084 μM and 0.086 μM, respectively. The higher binding capability of HA and FA is mainly attributed to their richer functional groups, lower zeta potential (HA/FA = -15.92/-13.54 mV) and the bigger molecular size (HA/FA = 24668/27750 nm). IS significantly inhibits the binding interaction by compressing the molecular structure and the surface electric double layer, while pH had a weak effect. By combining the Donnan model and the multiple linear regression analysis, a modified Karickhoff model was established to effectively predict the binding performance of DOM under different IS (0.001-0.1 M) and pH (5.0-9.0) conditions, and the R2 of linear fitting between experiment-measured logKDOC and model-calculated logKOC were 0.94 for HA and 0.91 for FA. This finding provides a theoretical basis for characterizing and predicting the binding performance of various DOMs to residual micropollutants in the natural water environment.
Keywords: Dissolved organic matters; Ionic strength; Karickhoff model; Tetracycline; pH.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Figures





Similar articles
-
Interactions between the antibiotic tetracycline and humic acid: Examination of the binding sites, and effects of complexation on the oxidation of tetracycline.Water Res. 2021 Sep 1;202:117379. doi: 10.1016/j.watres.2021.117379. Epub 2021 Jun 22. Water Res. 2021. PMID: 34246001
-
Insight into interactions of polystyrene microplastics with different types and compositions of dissolved organic matter.Sci Total Environ. 2022 Jun 10;824:153883. doi: 10.1016/j.scitotenv.2022.153883. Epub 2022 Feb 16. Sci Total Environ. 2022. PMID: 35182636
-
Adsorption of dissolved organic matter (DOM) on polystyrene microplastics in aquatic environments: Kinetic, isotherm and site energy distribution analysis.Ecotoxicol Environ Saf. 2020 Jul 15;198:110658. doi: 10.1016/j.ecoenv.2020.110658. Epub 2020 Apr 24. Ecotoxicol Environ Saf. 2020. PMID: 32339926
-
Characterization of isolated fractions of dissolved organic matter from natural waters and a wastewater effluent.Water Res. 2001 Mar;35(4):985-96. doi: 10.1016/s0043-1354(00)00350-x. Water Res. 2001. PMID: 11235894
-
Insights into the impacts of dissolved organic matter of different origins on bioaccumulation and translocation of per- and polyfluoroalkyl substances (PFASs) in wheat.Environ Pollut. 2022 Jan 15;293:118604. doi: 10.1016/j.envpol.2021.118604. Epub 2021 Nov 30. Environ Pollut. 2022. PMID: 34856244
Cited by
-
How aromatic dissolved organic matter differs in competitiveness against organic micropollutant adsorption.Environ Sci Ecotechnol. 2024 Jan 27;21:100392. doi: 10.1016/j.ese.2024.100392. eCollection 2024 Sep. Environ Sci Ecotechnol. 2024. PMID: 38434492 Free PMC article.
-
The Influence Mechanism of Dissolved Organic Matter on the Photocatalytic Oxidation of Pharmaceuticals and Personal Care Products.Molecules. 2025 May 22;30(11):2266. doi: 10.3390/molecules30112266. Molecules. 2025. PMID: 40509152 Free PMC article. Review.
-
Tailoring a novel hierarchical cheese-like porous biochar from algae residue to boost sulfathiazole removal.Environ Sci Ecotechnol. 2022 Mar 6;10:100168. doi: 10.1016/j.ese.2022.100168. eCollection 2022 Apr. Environ Sci Ecotechnol. 2022. PMID: 36159736 Free PMC article.
References
-
- Mo W.Y., Chen Z., Leung H.M., Leung A.O. Application of veterinary antibiotics in China's aquaculture industry and their potential human health risks. Environ. Sci. Pollut. Res. Int. 2017;24:8978–8989. - PubMed
-
- Chen Q., Yi P., Dong W., Chen Y.H., He L.P., Pan B. Decisive role of adsorption affinity in antibiotic adsorption on a positively charged MnFe2O4@CAC hybrid. Sci. Total Environ. 2020:745. - PubMed
-
- Zhang Q.Q., Ying G.G., Pan C.G., Liu Y.S., Zhao J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015;49:6772–6782. - PubMed
-
- Chi Z.X., Liu R.T., You H., Wang D.L. Binding of the veterinary drug tetracycline to bovine hemoglobin and toxicological implications. J. Environ. Sci. Health. 2014;49:978–984. - PubMed
-
- Rutgersson C., Fick J., Marathe N., Kristiansson E., Janzon A., Angelin M., Johansson A., Shouche Y., Flach C.F., Larsson D.G.J. Fluoroquinolones and qnr genes in sediment, water, soil, and human fecal flora in an environment polluted by manufacturing discharges. Environ. Sci. Technol. 2014;48:7825–7832. - PubMed
LinkOut - more resources
Full Text Sources

