Synthesis and evaluation of anticancer activity of quillaic acid derivatives: A cell cycle arrest and apoptosis inducer through NF- κ B and MAPK pathways
- PMID: 36157038
- PMCID: PMC9490060
- DOI: 10.3389/fchem.2022.951713
Synthesis and evaluation of anticancer activity of quillaic acid derivatives: A cell cycle arrest and apoptosis inducer through NF- κ B and MAPK pathways
Abstract
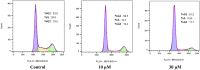
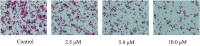
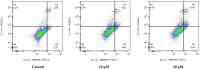
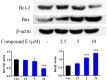
A series of quillaic acid derivatives with different substituents on the 28-carboxyl group were designed and synthesized. Five human cancer cell lines (HCT116, BEL7402, HepG2, SW620, and MCF-7) were evaluated for their antitumor activity in vitro. Some of the tested derivatives showed improved antiproliferative activity compared to the lead compound, quillaic acid. Among them, compound E (IC50 = 2.46 ± 0.44 μM) showed the strongest antiproliferative activity against HCT116 cells; compared with quillaic acid (IC50 > 10 μM), its efficacy against HCT116 cancer cells was approximately 4-fold higher than that of quillaic acid. Compound E also induces cell cycle arrest and apoptosis by modulating NF-κB and MAPK pathways. Therefore, the development of compound E is certainly valuable for anti-tumor applications.
Keywords: Western blot; antitumor; apoptosis; cell-cycle arrest; quillaic acid.
Copyright © 2022 Huang, Zhang, Deng, Wu, Guo, Lee, Chen, Shen, Jin and Quan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Synthesis, and evaluation of in vitro and in vivo anticancer activity of 14-substituted oridonin analogs: A novel and potent cell cycle arrest and apoptosis inducer through the p53-MDM2 pathway.Eur J Med Chem. 2019 Jul 1;173:15-31. doi: 10.1016/j.ejmech.2019.04.005. Epub 2019 Apr 6. Eur J Med Chem. 2019. PMID: 30981113
-
Altersolanol B, a fungal tetrahydroanthraquinone, inhibits the proliferation of estrogen receptor-expressing (ER+) human breast adenocarcinoma by modulating PI3K/AKT, p38/ERK MAPK and associated signaling pathways.Chem Biol Interact. 2022 May 25;359:109916. doi: 10.1016/j.cbi.2022.109916. Epub 2022 Mar 26. Chem Biol Interact. 2022. PMID: 35346647
-
Design, synthesis, in vitro antiproliferative activity and apoptosis-inducing studies of 1-(3',4',5'-trimethoxyphenyl)-3-(2'-alkoxycarbonylindolyl)-2-propen-1-one derivatives obtained by a molecular hybridisation approach.J Enzyme Inhib Med Chem. 2018 Dec;33(1):1225-1238. doi: 10.1080/14756366.2018.1493473. J Enzyme Inhib Med Chem. 2018. PMID: 30141353 Free PMC article.
-
Antiproliferative Properties of 7,8-Ethylene Diamine Chelator-Lipophilic Fluoroquinolone Derivatives Against Colorectal Cancer Cell Lines.Anticancer Agents Med Chem. 2022;22(5):1012-1028. doi: 10.2174/1871520621666210623111744. Anticancer Agents Med Chem. 2022. PMID: 34165411
-
Synthesis, Anticancer Assessment, and Molecular Docking of Novel Chalcone-Thienopyrimidine Derivatives in HepG2 and MCF-7 Cell Lines.Oxid Med Cell Longev. 2021 Dec 28;2021:4759821. doi: 10.1155/2021/4759821. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 35003514 Free PMC article.
Cited by
-
UPLC-QToF-MS/MS screening and characterization of Symphorema polyandrum Wight and in vitro assessment of its antioxidant, anticancer, and anti-inflammatory potential.3 Biotech. 2024 Dec;14(12):298. doi: 10.1007/s13205-024-04144-x. Epub 2024 Nov 12. 3 Biotech. 2024. PMID: 39544488
References
-
- Balaraju V., Kalyani S., Sridhar G., Laxminarayana E. (2021). Design, synthesis and biological assessment of 1, 3, 4-oxadiazole incorporated oxazole-triazine derivatives as anticancer agents. Chem. Data Collect. 33, 100695. 10.1016/j.cdc.2021.100695 - DOI
-
- Boke Sarikahya N., Goren A. C., Sumer Okkali G., Coven F. O., Orman B., Kirci D., et al. (2021). Chemical composition and biological activities of propolis samples from different geographical regions of Turkey. Phytochem. Lett. 44, 129–136. 10.1016/j.phytol.2021.06.008 - DOI
LinkOut - more resources
Full Text Sources

