Cost-Effectiveness Analysis of the Oncotype DX Breast Recurrence Score® Test in Node-Negative Early Breast Cancer
- PMID: 36157054
- PMCID: PMC9505370
- DOI: 10.2147/CEOR.S360049
Cost-Effectiveness Analysis of the Oncotype DX Breast Recurrence Score® Test in Node-Negative Early Breast Cancer
Abstract
Background: The 21-gene assay (the Oncotype DX Breast Recurrence Score® test) is a validated multigene assay which produces the Recurrence Score® result (RS) to inform decisions on the use of adjuvant chemotherapy in human epidermal growth factor receptor 2-negative (HER2-), hormone receptor positive (HR+) early invasive breast cancer. A model-based economic evaluation estimated the cost-effectiveness of the 21-gene assay against the use of clinical risk tools alone based on the latest evidence from prospective studies.
Methods: The proportion of patients assigned to chemotherapy conditional on their RS result was obtained from retrospective data from the Clalit registry. The probability of distant recurrence with endocrine and chemo-endocrine therapy conditional on RS result was obtained from TAILORx and NSABP B-20 trials. The cost-effectiveness of the 21-gene assay compared to using clinical risk tools alone was estimated in terms of cost per quality-adjusted life-year (QALY) over a lifetime horizon.
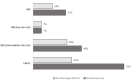
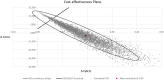
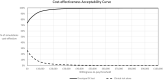
Results: The 21-gene assay was more effective (0.17 more quality-adjusted life years) at a lower cost (-£519) over a lifetime compared to clinical risk alone. The model results were sensitive to assumptions around the magnitude of benefit of chemotherapy in the high RS result subgroup. Other assumptions underpinning the model, such as the proportion of patients assigned to chemotherapy in the low and mid-range RS result subgroups and long-term distant recurrence probabilities, had a smaller impact on the results.
Conclusion: The analysis showed that the cost-effectiveness of the 21-gene assay is sensitive to assumptions for chemotherapy sparing for patients with RS 0-25 whose outcomes with endocrine therapy are no worse compared to chemotherapy-assigned patients, and a chemotherapy benefit in the RS 26-100 group. Future studies need to incorporate a wider set of tumour profiling tests other than the 21-gene assay to allow a direct comparison of their cost-effectiveness.
Keywords: 21-gene assay; breast cancer; chemotherapy; cost-effectiveness; multigene assay; the Oncotype DX test.
© 2022 Berdunov et al.
Conflict of interest statement
Steve Millen, Andrew Paramore, Sarah Reynia and Nina Fryer are employees and stockholders of Exact Sciences. The authors report no other conflicts of interest in this work.
Figures

References
-
- Cancer Research UK. Breast cancer incidence (invasive) statistics. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/s.... Accessed September 15, 2020.
-
- Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol. 2012;13(4):e148–e160. doi: 10.1016/S1470-2045(11)70383-7 - DOI - PubMed
-
- National Institute for Health and Care Excellence. Early and locally advanced breast cancer: diagnosis and management; 2018. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

