Gut microbiota, inflammatory bowel disease and colorectal cancer
- PMID: 36157114
- PMCID: PMC9403435
- DOI: 10.3748/wjg.v28.i30.4053
Gut microbiota, inflammatory bowel disease and colorectal cancer
Abstract
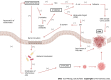
The gut microbiota is a complex community of microorganisms that inhabit the digestive tracts of humans, living in symbiosis with the host. Dysbiosis, characterized by an imbalance between the beneficial and opportunistic gut microbiota, is associated with several gastrointestinal disorders, such as irritable bowel syndrome (IBS); inflammatory bowel disease (IBD), represented by ulcerative colitis and Crohn's disease; and colorectal cancer (CRC). Dysbiosis can disrupt the mucosal barrier, resulting in perpetuation of inflammation and carcinogenesis. The increase in some specific groups of harmful bacteria, such as Escherichia coli (E. coli) and enterotoxigenic Bacteroides fragilis (ETBF), has been associated with chronic tissue inflammation and the release of pro-inflammatory and carcinogenic mediators, increasing the chance of developing CRC, following the inflammation-dysplasia-cancer sequence in IBD patients. Therefore, the aim of the present review was to analyze the correlation between changes in the gut microbiota and the development and maintenance of IBD, CRC, and IBD-associated CRC. Patients with IBD and CRC have shown reduced bacterial diversity and abundance compared to healthy individuals, with enrichment of Firmicute sand Bacteroidetes. Specific bacteria are also associated with the onset and progression of CRC, such as Fusobacterium nucleatum, E. coli, Enterococcus faecalis, Streptococcus gallolyticus, and ETBF. Future research can evaluate the advantages of modulating the gut microbiota as preventive measures in CRC high-risk patients, directly affecting the prognosis of the disease and the quality of life of patients.
Keywords: Colorectal cancer; Crohn’s disease; Dysbiosis; Gut microbiota; Inflammatory bowel disease; Ulcerative colitis.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: There are no conflicts of interest to report.
Figures
References
-
- Michaudel C, Sokol H. The Gut Microbiota at the Service of Immunometabolism. Cell Metab. 2020;32:514–523. - PubMed
-
- Kc D, Sumner R, Lippmann S. Gut microbiota and health. Postgrad Med. 2020;132:274. - PubMed
-
- Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek. 2020;113:2019–2040. - PubMed
-
- Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

