Basal insulin intensification with GLP-1RA and dual GIP and GLP-1RA in patients with uncontrolled type 2 diabetes mellitus: A rapid review of randomized controlled trials and meta-analysis
- PMID: 36157450
- PMCID: PMC9494570
- DOI: 10.3389/fendo.2022.920541
Basal insulin intensification with GLP-1RA and dual GIP and GLP-1RA in patients with uncontrolled type 2 diabetes mellitus: A rapid review of randomized controlled trials and meta-analysis
Abstract
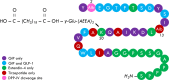
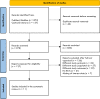
Tirzepatide, a dual agonist of Glucose-Dependent Insulinotropic Polypeptide (GIP) and Glucagon-Like Peptide 1 (GLP-1) receptors, improved glucose control and reduced body weight in different therapeutic approaches. Herein, we overviewed the role of GIP and GLP-1 in the pathophysiology of type 2 diabetes and systematically reviewed the efficacy and safety of injectable incretin-based therapy added to basal insulin in light of the results of the SURPASS-5 trial. We identified eleven randomized clinical trials. GLP-1 receptor agonists (GLP-1RAs) or Tirzepatide added to basal insulin than rigorously titrated basal insulin significantly ameliorates glucose control (Δ HbA1c = -1%, 95% CI -1.25; -0.74, I2 94%; Δ FPG = -14.6 mg/dL, 95% CI -21.6-; -7.6, I2 90%; chance to achieve HbA1c <7% = RR 2.62, 95% CI 2.10; 3.26, I2 89%), reduces body weight (Δ = -3.95 kg, 95% CI -5.1, -2.79, I2 96%) without increasing the risk of hypoglycemia (RR = 1.01, 95% CI 0.86; 1.18, I2 7.7%). Tirzepatide provides an impressive weight loss exceeding that observed with GLP-1RAs. Injectable incretin-based therapy plus basal insulin remains a potent and safe therapeutic approach in uncontrolled type 2 diabetes patients previously treated with basal insulin alone. Tirzepatide is expected to ameliorate the management of "diabesity" in this usually difficult-to-treat cluster of patients.
Keywords: GIP, GLP-1; basal insulin; body weight; hypoglycemia; obesity; tirzepatide; type 2 diabetes.
Copyright © 2022 Lisco, De Tullio, Disoteo, De Geronimo, Piazzolla, De Pergola, Giagulli, Jirillo, Guastamacchia, Sabbà and Triggiani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Meta-analysis of head-to-head clinical trials comparing incretin-based glucose-lowering medications and basal insulin: An update including recently developed glucagon-like peptide-1 (GLP-1) receptor agonists and the glucose-dependent insulinotropic polypeptide/GLP-1 receptor co-agonist tirzepatide.Diabetes Obes Metab. 2023 May;25(5):1361-1371. doi: 10.1111/dom.14988. Epub 2023 Feb 8. Diabetes Obes Metab. 2023. PMID: 36700380
-
Tirzepatide, a dual GIP/GLP-1 receptor co-agonist for the treatment of type 2 diabetes with unmatched effectiveness regrading glycaemic control and body weight reduction.Cardiovasc Diabetol. 2022 Sep 1;21(1):169. doi: 10.1186/s12933-022-01604-7. Cardiovasc Diabetol. 2022. PMID: 36050763 Free PMC article. Review.
-
The dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist tirzepatide: a novel cardiometabolic therapeutic prospect.Cardiovasc Diabetol. 2021 Nov 24;20(1):225. doi: 10.1186/s12933-021-01412-5. Cardiovasc Diabetol. 2021. PMID: 34819089 Free PMC article.
-
Incretin hormones and type 2 diabetes.Diabetologia. 2023 Oct;66(10):1780-1795. doi: 10.1007/s00125-023-05956-x. Epub 2023 Jul 11. Diabetologia. 2023. PMID: 37430117 Free PMC article. Review.
-
Achievement of glycaemic targets with weight loss and without hypoglycaemia in type 2 diabetes with the once-weekly glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist tirzepatide: A post hoc analysis of the SURPASS-1 to -5 studies.Diabetes Obes Metab. 2023 Apr;25(4):965-974. doi: 10.1111/dom.14943. Epub 2022 Dec 27. Diabetes Obes Metab. 2023. PMID: 36514843 Clinical Trial.
Cited by
-
Dopamine in the Regulation of Glucose Homeostasis, Pathogenesis of Type 2 Diabetes, and Chronic Conditions of Impaired Dopamine Activity/Metabolism: Implication for Pathophysiological and Therapeutic Purposes.Biomedicines. 2023 Nov 7;11(11):2993. doi: 10.3390/biomedicines11112993. Biomedicines. 2023. PMID: 38001993 Free PMC article. Review.
-
Tirzepatide: A Systematic Update.Int J Mol Sci. 2022 Nov 23;23(23):14631. doi: 10.3390/ijms232314631. Int J Mol Sci. 2022. PMID: 36498958 Free PMC article. Review.
-
Insulin Therapy for the Management of Diabetes Mellitus: A Narrative Review of Innovative Treatment Strategies.Diabetes Ther. 2023 Nov;14(11):1801-1831. doi: 10.1007/s13300-023-01468-4. Epub 2023 Sep 22. Diabetes Ther. 2023. PMID: 37736787 Free PMC article. Review.
-
Bridging the gap between GLP1-receptor agonists and cardiovascular outcomes: evidence for the role of tirzepatide.Cardiovasc Diabetol. 2024 Jul 10;23(1):242. doi: 10.1186/s12933-024-02319-7. Cardiovasc Diabetol. 2024. PMID: 38987789 Free PMC article. Review.
-
The rise of weekly insulins: addressing the challenges of type 2 diabetes care in Brazil.Diabetol Metab Syndr. 2025 Jan 15;17(1):14. doi: 10.1186/s13098-024-01560-0. Diabetol Metab Syndr. 2025. PMID: 39810242 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

