PGRN exacerbates the progression of non-small cell lung cancer via PI3K/AKT/Bcl-2 antiapoptotic signaling
- PMID: 36157487
- PMCID: PMC9485207
- DOI: 10.1016/j.gendis.2021.05.005
PGRN exacerbates the progression of non-small cell lung cancer via PI3K/AKT/Bcl-2 antiapoptotic signaling
Abstract
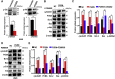
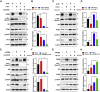
Progranulin (PGRN) is a growth factor that is involved in the progression of multiple tumors. However, the effects and molecular mechanisms by which PGRN induces lung cancer remain unclear. The expression level of PGRN was analyzed by conducting immunohistochemistry of the histological sections of lung tissues from non-small-cell lung carcinoma (NSCLC) patients. The proliferation, apoptosis, migration, and invasion of NSCLC cells were assessed by the MTT assay, Western blot, degree of wound healing, and Transwell assays. A nude mouse xenograft model was used to validate the role of PGRN in vivo. The expression level of PGRN was higher in male patients with lung adenocarcinoma than in those with lung squamous cell carcinoma; by contrast, no difference was observed in female patients. The overexpression of PGRN promoted the proliferation and anti-apoptosis of H520 (derived from lung squamous cell carcinoma) cells, whereas knockdown of PGRN inhibited the proliferation and anti-apoptosis of A549 (derived from lung adenocarcinoma) cells. Copanlisib (targeting PI3K) inhibited the increase in the expression of cell anti-apoptosis marker Bcl-2 induced by rhPGRN protein; the PI3K agonist 740 Y-P partially reversed the decrease in Bcl-2 expression induced by PGRN deficiency in both A549 and H520 cells. PGRN increased the expression of Ki-67, PCNA, and Bcl-2 in vivo. PGRN inhibited cell apoptosis depending on the PI3K/Akt/Bcl-2 signaling axis; PGRN positivity correlated with lung adenocarcinoma. PGRN is a potential biomarker for the treatment and diagnosis of NSCLC, especially in lung adenocarcinoma.
Keywords: Ad, Adenovirus; Bcl-2; Cell apoptosis; DMSO, Dimethyl sulfoxide; FBS, Fetal Bovine Serum; IHC, immunohistochemistry; MTT, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide; NSCLC; NSCLC, Non-small cell lung cancer; PBS, phosphate buffer saline; PGRN; PI3K/Akt.
© 2021 Chongqing Medical University. Production and hosting by Elsevier B.V.
Figures



Similar articles
-
Glycogen phosphorylase B promotes cell proliferation and migration through PI3K/AKT pathway in non-small cell lung cancer.Exp Lung Res. 2021 Apr;47(3):111-120. doi: 10.1080/01902148.2020.1864065. Epub 2020 Dec 18. Exp Lung Res. 2021. PMID: 33336613
-
Apoptosis induced by the methanol extract of Salvia miltiorrhiza Bunge in non-small cell lung cancer through PTEN-mediated inhibition of PI3K/Akt pathway.J Ethnopharmacol. 2017 Mar 22;200:107-116. doi: 10.1016/j.jep.2016.12.051. Epub 2017 Jan 12. J Ethnopharmacol. 2017. PMID: 28088493
-
Effect of microRNA-135a on Cell Proliferation, Migration, Invasion, Apoptosis and Tumor Angiogenesis Through the IGF-1/PI3K/Akt Signaling Pathway in Non-Small Cell Lung Cancer.Cell Physiol Biochem. 2017;42(4):1431-1446. doi: 10.1159/000479207. Epub 2017 Jul 17. Cell Physiol Biochem. 2017. PMID: 28715819
-
Silencing long non-coding RNA ROR improves sensitivity of non-small-cell lung cancer to cisplatin resistance by inhibiting PI3K/Akt/mTOR signaling pathway.Tumour Biol. 2017 May;39(5):1010428317697568. doi: 10.1177/1010428317697568. Tumour Biol. 2017. PMID: 28459375
-
Long noncoding RNA Linc00210 promotes non-small cell lung cancer progression via sponging miR-16-5p/PTK2 axis.Eur Rev Med Pharmacol Sci. 2020 Sep;24(18):9438-9452. doi: 10.26355/eurrev_202009_23029. Eur Rev Med Pharmacol Sci. 2020. PMID: 33015786
Cited by
-
Baicalin Inhibits FIPV Infection In Vitro by Modulating the PI3K-AKT Pathway and Apoptosis Pathway.Int J Mol Sci. 2024 Sep 14;25(18):9930. doi: 10.3390/ijms25189930. Int J Mol Sci. 2024. PMID: 39337417 Free PMC article.
-
Nanomedicine Strategies for Management of Drug Resistance in Lung Cancer.Int J Mol Sci. 2022 Feb 6;23(3):1853. doi: 10.3390/ijms23031853. Int J Mol Sci. 2022. PMID: 35163777 Free PMC article. Review.
-
FANCI is involved in the malignant progression of glioma cells by regulating the Akt/Bcl-2 signaling pathway.Discov Oncol. 2025 May 13;16(1):753. doi: 10.1007/s12672-025-02284-x. Discov Oncol. 2025. PMID: 40358883 Free PMC article.
-
Network Pharmacology and in vitro Experimental Verification on Intervention of Oridonin on Non-Small Cell Lung Cancer.Chin J Integr Med. 2025 Apr;31(4):347-356. doi: 10.1007/s11655-024-4116-7. Epub 2024 Sep 27. Chin J Integr Med. 2025. PMID: 39331210
-
Chondroitin Polymerizing Factor (CHPF) promotes cell proliferation and tumor growth in human osteosarcoma by inhibiting SKP2's ubiquitination while activating the AKT pathway.Genes Dis. 2022 Aug 6;10(5):2125-2136. doi: 10.1016/j.gendis.2022.06.010. eCollection 2023 Sep. Genes Dis. 2022. PMID: 37492722 Free PMC article.
References
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Chen W., Zheng R., Zhang S., et al. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017;401:63–71. - PubMed
-
- Hirsch F.R., Scagliotti G.V., Mulshine J.L., et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311. - PubMed
-
- Songsrirote K., Li Z., Ashford D., Bateman A., Thomas-Oates J. Development and application of mass spectrometric methods for the analysis of progranulin N-glycosylation. J Proteomics. 2010;73(8):1479–1490. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

