Anti-viral efficacy of a next-generation CD4-binding site bNAb in SHIV-infected animals in the absence of anti-drug antibody responses
- PMID: 36157588
- PMCID: PMC9490026
- DOI: 10.1016/j.isci.2022.105067
Anti-viral efficacy of a next-generation CD4-binding site bNAb in SHIV-infected animals in the absence of anti-drug antibody responses
Abstract
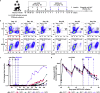
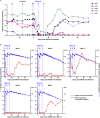
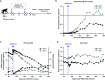
Broadly neutralizing antibodies (bNAbs) against HIV-1 are promising immunotherapeutic agents for treatment of HIV-1 infection. bNAbs can be administered to SHIV-infected rhesus macaques to assess their anti-viral efficacy; however, their delivery into macaques often leads to rapid formation of anti-drug antibody (ADA) responses limiting such assessment. Here, we depleted B cells in five SHIV-infected rhesus macaques by pretreatment with a depleting anti-CD20 antibody prior to bNAb infusions to reduce ADA. Peripheral B cells were depleted following anti-CD20 infusions and remained depleted for at least 9 weeks after the 1st anti-CD20 infusion. Plasma viremia dropped by more than 100-fold in viremic animals after the initial bNAb treatment. No significant humoral ADA responses were detected for as long as B cells remained depleted. Our results indicate that transient B cell depletion successfully inhibited emergence of ADA and improved the assessment of anti-viral efficacy of a bNAb in a SHIV-infected rhesus macaque model.
Keywords: Drugs; Immunology; Virology.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Rapid Elimination of Broadly Neutralizing Antibodies Correlates with Treatment Failure in the Acute Phase of Simian-Human Immunodeficiency Virus Infection.J Virol. 2019 Sep 30;93(20):e01077-19. doi: 10.1128/JVI.01077-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31375583 Free PMC article.
-
Anti-Drug Antibodies in Pigtailed Macaques Receiving HIV Broadly Neutralising Antibody PGT121.Front Immunol. 2021 Nov 11;12:749891. doi: 10.3389/fimmu.2021.749891. eCollection 2021. Front Immunol. 2021. PMID: 34867979 Free PMC article.
-
Introduction of the YTE mutation into the non-immunogenic HIV bnAb PGT121 induces anti-drug antibodies in macaques.PLoS One. 2019 Feb 20;14(2):e0212649. doi: 10.1371/journal.pone.0212649. eCollection 2019. PLoS One. 2019. PMID: 30785963 Free PMC article.
-
Development of Broadly Neutralizing Antibodies and Their Mapping by Monomeric gp120 in Human Immunodeficiency Virus Type 1-Infected Humans and Simian-Human Immunodeficiency Virus SHIVSF162P3N-Infected Macaques.J Virol. 2016 Mar 28;90(8):4017-4031. doi: 10.1128/JVI.02898-15. Print 2016 Apr. J Virol. 2016. PMID: 26842476 Free PMC article.
-
Importance of Fc-mediated functions of anti-HIV-1 broadly neutralizing antibodies.Retrovirology. 2018 Aug 22;15(1):58. doi: 10.1186/s12977-018-0438-x. Retrovirology. 2018. PMID: 30134945 Free PMC article. Review.
Cited by
-
Trispecific antibody targeting HIV-1 and T cells activates and eliminates latently-infected cells in HIV/SHIV infections.Nat Commun. 2023 Jun 22;14(1):3719. doi: 10.1038/s41467-023-39265-z. Nat Commun. 2023. PMID: 37349337 Free PMC article.
-
Adeno-associated virus-vectored delivery of HIV biologics: the promise of a "single-shot" functional cure for HIV infection.J Virus Erad. 2023 Feb 17;9(1):100316. doi: 10.1016/j.jve.2023.100316. eCollection 2023 Mar. J Virus Erad. 2023. PMID: 36915910 Free PMC article.
References
-
- Bauer A.M., Bar K.J. Advances in simian--human immunodeficiency viruses for nonhuman primate studies of HIV prevention and cure. Curr. Opin. HIV AIDS. 2020;15:275–281. - PubMed
-
- Bolton D.L., Pegu A., Wang K., McGinnis K., Nason M., Foulds K., Letukas V., Schmidt S.D., Chen X., Todd J.P., et al. Human immunodeficiency virus type 1 monoclonal antibodies suppress acute simian-human immunodeficiency virus viremia and limit seeding of cell-associated viral reservoirs. J. Virol. 2015;90:1321–1332. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

