Electronic Properties and Electrocatalytic Water Splitting Activity for Precious-Metal-Adsorbed Silicene with Nonmetal Doping
- PMID: 36157726
- PMCID: PMC9494430
- DOI: 10.1021/acsomega.2c03388
Electronic Properties and Electrocatalytic Water Splitting Activity for Precious-Metal-Adsorbed Silicene with Nonmetal Doping
Abstract
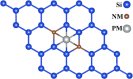
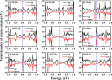
Since nonmetal (NM)-doped two-dimensional (2D) materials can effectively modulate their physical properties and chemical activities, they have received a lot of attention from researchers. Therefore, the stability, electronic properties, and electrocatalytic water splitting activity of precious-metal (PM)-adsorbed silicene doped with two NM atoms are investigated based on density functional theory (DFT) in this paper. The results show that NM doping can effectively improve the stability of PM-adsorbed silicene and exhibit rich electronic properties. Meanwhile, by comparing the free energies of the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) intermediates of 15 more stable NM-doped systems, it can be concluded that the electrocatalytic water splitting activity of the NM-doped systems is more influenced by the temperature. Moreover, the Si-S2-Ir-doped system exhibits good HER performance when the temperature is 300 K, while the Si-N2-Pt-doped system shows excellent OER activity. Our theoretical study shows that NM doping can effectively promote the stability and electrocatalytic water splitting of PM-adsorbed silicene, which can help in the application of silicene in electrocatalytic water splitting.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
-
- Chen H.; Cong T. N.; Yang W.; Tan C.; Li Y.; Ding Y. Progress in Electrical Energy Storage System: A Critical Review. Prog. Nat. Sci. 2009, 19, 291–312. 10.1016/j.pnsc.2008.07.014. - DOI
-
- Momirlan M.; Veziroglu T. N. The Properties of Hydrogen as Fuel Tomorrow in Sustainable Energy System for a Cleaner Planet. Int. J. Hydrogen Energy 2005, 30, 795–802. 10.1016/j.ijhydene.2004.10.011. - DOI
-
- Nørskov J. K.; Rossmeisl J.; Logadottir A.; Lindqvist L.; Kitchin J. R.; Bligaard T.; Jónsson H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. 10.1021/jp047349j. - DOI
-
- Nørskov J. K.; Bligaard T.; Logadottir A.; Kitchin J. R.; Chen J. G.; Pandelov S.; Stimming U. Trends in the Exchange Current for Hydrogen Evolution. J. Electrochem. Soc. 2005, 152, J23–J26. 10.1149/1.1856988. - DOI
LinkOut - more resources
Full Text Sources

