Temperature and Pressure-Dependent Rate Constants for the Reaction of the Propargyl Radical with Molecular Oxygen
- PMID: 36157753
- PMCID: PMC9494672
- DOI: 10.1021/acsomega.2c04316
Temperature and Pressure-Dependent Rate Constants for the Reaction of the Propargyl Radical with Molecular Oxygen
Erratum in
-
Correction to Temperature and Pressure-Dependent Rate Constants for the Reaction of Propargyl Radical with Molecular Oxygen.ACS Omega. 2022 Sep 30;7(40):36048. doi: 10.1021/acsomega.2c05858. eCollection 2022 Oct 11. ACS Omega. 2022. PMID: 36249374 Free PMC article.
Abstract
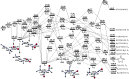
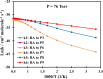
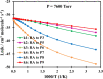
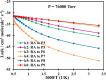
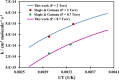
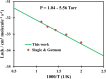
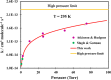
Ab initio CCSD(T)/CBS(T,Q,5)//B3LYP/6-311++G(3df,2p) calculations have been conducted to map the C3H3O2 potential energy surface. The temperature- and pressure-dependent reaction rate constants have been calculated using the Rice-Ramsperger-Kassel-Marcus Master Equation model. The calculated results indicate that the prevailing reaction channels lead to CH3CO + CO and CH2CO + HCO products. The branching ratios of CH3CO + CO and CH2CO + HCO increase both from 18 to 29% with reducing temperatures in the range of 300-2000 K, whereas CCCHO + H2O (0-10%) and CHCCO + H2O (0-17%) are significant minor products. The desirable products OH and H2O have been found for the first time. The individual rate constant of the C3H3 + O2 → CH2CO + HCO channel, 4.8 × 10-14 exp[(-2.92 kcal·mol-1)/(RT)], is pressure independent; however, the total rate constant, 2.05 × 10-14 T0.33 exp[(-2.8 ± 0.03 kcal·mol-1)/(RT)], of the C3H3 + O2 reaction leading to the bimolecular products strongly depends on pressure. At P = 0.7-5.56 Torr, the calculated rate constants of the reaction agree closely with the laboratory values measured by Slagle and Gutman [Symp. (Int.) Combust.1988, 21, 875-883] with the uncertainty being less than 7.8%. At T < 500 K, the C3H3 + O2 reaction proceeds by simple addition, making an equilibrium of C3H3 + O2 ⇌ C3H3O2. The calculated equilibrium constants, 2.60 × 10-16-8.52 × 10-16 cm3·molecule-1, were found to be in good agreement with the experimental data, being 2.48 × 10-16-8.36 × 10-16 cm3·molecule-1. The title reaction is concluded to play a substantial role in the oxidation of the five-member radicals and the present results corroborate the assertion that molecular oxygen is an efficient oxidizer of the propargyl radical.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Lander D. R.; Unfried K. G.; Stephens J. W.; Glass G. P.; Curl R. F. Reaction mechanism of ethynyl radical+ oxygen. J. Phys. Chem. A 1989, 93, 4109–4116. 10.1021/j100347a047. - DOI
-
- Lander D. R.; Unfriend K. G.; Glass G. P.; Curl R. F. Rate constant measurements of ethynyl radical with methane, ethane, ethylene, deuterium, and carbon monoxide. J. Phys. Chem. B 1990, 94, 7759–7763. 10.1021/j100383a003. - DOI
-
- Miller J. A.; Melius C. F. Kinetic and thermodynamic issues in the formation of aromatic compounds in flames of aliphatic fuels. Combust. Flame 1992, 91, 21–39. 10.1016/0010-2180(92)90124-8. - DOI
-
- Kern R. D.; Singh H. J.; Wu C. H. Thermal decomposition of 1, 2 butadiene. Int. J. Chem. Kinet. 1988, 20, 731–747. 10.1002/kin.550200907. - DOI
-
- D’Anna A.; Violi A.; D’Allessio A. Modeling the rich combustion of aliphatic hydrocarbons. Combust. Flame 2000, 121, 418–429. 10.1016/S0010-2180(99)00163-7. - DOI
LinkOut - more resources
Full Text Sources

