A one-pot electrochemical synthesis of 2-aminothiazoles from active methylene ketones and thioureas mediated by NH4I
- PMID: 36158175
- PMCID: PMC9490072
- DOI: 10.3762/bjoc.18.130
A one-pot electrochemical synthesis of 2-aminothiazoles from active methylene ketones and thioureas mediated by NH4I
Abstract
The electrochemical preparation of 2-aminothiazoles has been achieved by the reaction of active methylene ketones with thioureas assisted by ᴅʟ-alanine using NH4I as a redox mediator. The electrochemical protocol proceeds in an undivided cell equipped with graphite plate electrodes under constant current conditions. Various active methylene ketones, including β-keto ester, β-keto amide, β-keto nitrile, β-keto sulfone and 1,3-diketones, can be converted to the corresponding 2-aminothiazoles. Mechanistically, the in situ generated α-iodoketone was proposed to be the key active species.
Keywords: 2-aminothiazoles; electrosynthesis; halide ion; indirect electrolysis.
Copyright © 2022, Yang et al.
Figures


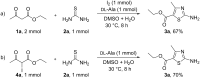

References
-
- de Souza M V N. J Sulfur Chem. 2005;26(4-5):429–449. doi: 10.1080/17415990500322792. - DOI
-
- Samadhiya P, Sharma R, Srivastava S K, Srivastava S D. J Serb Chem Soc. 2012;77:599–605. doi: 10.2298/jsc110616002s. - DOI
-
- Sarıgüney A B, Kocabaş E, Erci F, Torlak E, Coşkun A. J Heterocycl Chem. 2018;55:2107–2110. doi: 10.1002/jhet.3254. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
