Well-Defined Aryl-FeII Complexes in Cross-Coupling and C-H Activation Processes
- PMID: 36158566
- PMCID: PMC9490821
- DOI: 10.1021/acs.organomet.1c00100
Well-Defined Aryl-FeII Complexes in Cross-Coupling and C-H Activation Processes
Abstract
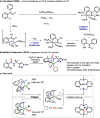
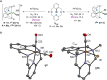
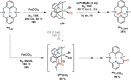
Herein we explore the intrinsic organometallic reactivity of iron embedded in a tetradentate N3C macrocyclic ligand scaffold that allows the stabilization of aryl-Fe species, which are key intermediates in Fe-catalyzed cross-coupling and C-H functionalization processes. This study covers C-H activation reactions using Me L H and FeCl2, biaryl C-C coupling product formation through reaction with Grignard reagents, and cross-coupling reactions using Me L Br or H L Br in combination with Fe0(CO)5. Synthesis under light irradiation and moderate heating (50 °C) affords the aryl-FeII complexes [FeII(Br)( Me L)(CO)] (1 Me ) and [FeII( H L)(CO)2]Br (1 H ). Exhaustive spectroscopic characterization of these rare low-spin diamagnetic species, including their crystal structures, allowed the investigation of their intrinsic reactivity.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Tamura M.; Kochi J. Iron catalysis in the reaction of grignard reagents with alkyl halides. J. Organomet. Chem. 1971, 31, 289–309. 10.1016/S0022-328X(00)86239-7. - DOI
-
- Tamura M.; Kochi J. K. Vinylation of Grignard reagents. Catalysis by iron. J. Am. Chem. Soc. 1971, 93, 1487–1489. 10.1021/ja00735a030. - DOI
-
- Cahiez G.; Avedissian H. Highly Stereo- and Chemoselective Iron-Catalyzed Alkenylation of Organomagnesium Compounds. Synthesis 1998, 1998, 1199–1205. 10.1055/s-1998-2135. - DOI
-
- Sears J. D.; Muñoz S. B. III; Daifuku S. L.; Shaps A. A.; Carpenter S. H.; Brennessel W. W.; Neidig M. L. The Effect of β-Hydrogen Atoms on Iron Speciation in Cross-Couplings with Simple Iron Salts and Alkyl Grignard Reagents. Angew. Chem., Int. Ed. 2019, 58, 2769–2773. 10.1002/anie.201813578. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
