Transcriptome changes in stages of non-alcoholic fatty liver disease
- PMID: 36158924
- PMCID: PMC9376779
- DOI: 10.4254/wjh.v14.i7.1382
Transcriptome changes in stages of non-alcoholic fatty liver disease
Abstract
Background: Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the United States and globally. The currently understood model of pathogenesis consists of a 'multiple hit' hypothesis in which environmental and genetic factors contribute to hepatic inflammation and injury.
Aim: To examine the genetic expression of NAFLD and non-alcoholic steatohepatitis (NASH) tissue samples to identify common pathways that contribute to NAFLD and NASH pathogenesis.
Methods: We employed the Search Tag Analyze Resource for Gene Expression Omnibus platform to search the The National Center for Biotechnology Information Gene Expression Omnibus to elucidate NAFLD and NASH pathology. For NAFLD, we conducted meta-analysis of data from 58 NAFLD liver biopsies and 60 healthy liver biopsies; for NASH, we analyzed 187 NASH liver biopsies and 154 healthy liver biopsies.
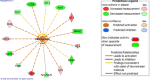
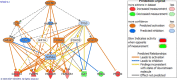
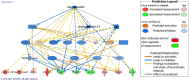
Results: Our results from the NAFLD analysis reinforce the role of altered metabolism, inflammation, and cell survival in pathogenesis and support recently described contributors to disease activity, such as altered androgen and long non-coding RNA activity. The top upstream regulator was found to be sterol regulatory element binding transcription factor 1 (SREBF1), a transcription factor involved in lipid homeostasis. Downstream of SREBF1, we observed upregulation in CXCL10, HMGCR, HMGCS1, fatty acid binding protein 5, paternally expressed imprinted gene 10, and downregulation of sex hormone-binding globulin and insulin-like growth factor 1. These molecular changes reflect low-grade inflammation secondary to accumulation of fatty acids in the liver. Our results from the NASH analysis emphasized the role of cholesterol in pathogenesis. Top canonical pathways, disease networks, and disease functions were related to cholesterol synthesis, lipid metabolism, adipogenesis, and metabolic disease. Top upstream regulators included pro-inflammatory cytokines tumor necrosis factor and IL1B, PDGF BB, and beta-estradiol. Inhibition of beta-estradiol was shown to be related to derangement of several cellular downstream processes including metabolism, extracellular matrix deposition, and tumor suppression. Lastly, we found riciribine (an AKT inhibitor) and ZSTK-474 (a PI3K inhibitor) as potential drugs that targeted the differential gene expression in our dataset.
Conclusion: In this study we describe several molecular processes that may correlate with NAFLD disease and progression. We also identified ricirbine and ZSTK-474 as potential therapy.
Keywords: AKT inhibitor; Bioinformatics; Non-alcoholic fatty liver disease; Non-alcoholic steatohepatitis; Therapy.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: None of the authors have any conflict of interest.
Figures


References
-
- Levene AP, Goldin RD. The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology. 2012;61:141–152. - PubMed
-
- Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. - PubMed
-
- Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. - PubMed
-
- Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

