Insights into induction of the immune response by the hepatitis B vaccine
- PMID: 36159002
- PMCID: PMC9453777
- DOI: 10.3748/wjg.v28.i31.4249
Insights into induction of the immune response by the hepatitis B vaccine
Abstract
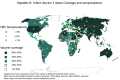
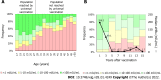
After more than four decades of hepatitis B virus (HBV) vaccine implementation, its safety and efficacy in preventing HBV infection have been proven and several milestones have been achieved. Most countries have included HBV immunization schedules in their health policies and progress has been made regarding universalization of the first HBV vaccine dose at birth. All of these actions have significantly contributed to reducing both the incidence of HBV infection and its related complications. However, there are still many drawbacks to overcome. The main concerns are the deficient coverage rate of the dose at birth and the large adult population that has not been reached timely by universal immunization. Additionally, the current most widely used second-generation vaccines do not induce protective immunity in 5% to 10% of the population, particularly in people over 40-years-old, obese (body mass index > 25 kg/m2), heavy smokers, and patients undergoing dialysis or infection with human immunodeficiency virus. Recently developed and approved novel vaccine formulations using more potent adjuvants or multiple antigens have shown better performance, particularly in difficult settings. These advances re-launch the expectations of achieving the World Health Organization's objective of completing hepatitis control by 2030.
Keywords: Antibodies; Hepatitis B virus; Immune response; Neutralizing; Vaccine.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Figures

References
-
- Beasley RP. Development of hepatitis B vaccine. JAMA. 2009;302:322–324. - PubMed
-
- Kao JT, Wang JH, Hung CH, Yen YH, Hung SF, Hu TH, Lee CM, Lu SN. Long-term efficacy of plasma-derived and recombinant hepatitis B vaccines in a rural township of Central Taiwan. Vaccine. 2009;27:1858–1862. - PubMed
-
- Hsu SH, Chih AH, Lee YC, Huang KC, Jan CF. Higher disappearance rate of anti-HBs in Taiwanese freshers neonatally vaccinated with recombinant yeast hepatitis B vaccine. Liver Int. 2017;37:1780–1787. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

