Effective gene therapy of Stargardt disease with PEG-ECO/ pGRK1-ABCA4-S/MAR nanoparticles
- PMID: 36159595
- PMCID: PMC9463552
- DOI: 10.1016/j.omtn.2022.08.026
Effective gene therapy of Stargardt disease with PEG-ECO/ pGRK1-ABCA4-S/MAR nanoparticles
Abstract
Stargardt disease (STGD) is the most common form of inherited retinal genetic disorders and is often caused by mutations in ABCA4. Gene therapy has the promise to effectively treat monogenic retinal disorders. However, clinically approved adeno-associated virus (AAV) vectors do not have a loading capacity for large genes, such as ABCA4. Self-assembly nanoparticles composed of (1-aminoethyl)iminobis[N-(oleoylcysteinyl-1-amino-ethyl)propionamide (ECO; a multifunctional pH-sensitive/ionizable amino lipid) and plasmid DNA produce gene transfection comparable with or better than the AAV2 capsid. Stable PEG-ECO/pGRK1-ABCA4-S/MAR nanoparticles produce specific and prolonged expression of ABCA4 in the photoreceptors of Abca4 -/- mice and significantly inhibit accumulation of toxic A2E in the eye. Multiple subretinal injections enhance gene expression and therapeutic efficacy with an approximately 69% reduction in A2E accumulation in Abca4 -/- mice after 3 doses. Very mild inflammation was observed after multiple injections of the nanoparticles. PEG-ECO/pGRK1-ABCA4-S/MAR nanoparticles are a promising non-viral mediated gene therapy modality for STGD type 1 (STGD1).
Keywords: ABCA4; ECO; MT: Delivery strategies; Stargardt disease; inherited retinal disorders; nanoparticles; non-viral gene therapy.
© 2022 The Author(s).
Conflict of interest statement
The gene therapy reported in this work was licensed to Helios BioPharmaceuticals for commercialization. Z.-R.L. may have ownership interest in the company.
Figures

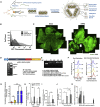

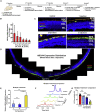
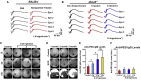
References
-
- Allikmets R., Shroyer N.F., Singh N., Seddon J.M., Lewis R.A., Bernstein P.S., Peiffer A., Zabriskie N.A., Li Y., Hutchinson A., et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277:1805–1807. - PubMed
-
- Molday R.S., Garces F.A., Scortecci J.F., Molday L.L. Structure and function of ABCA4 and its role in the visual cycle and Stargardt macular degeneration. Prog. Retin. Eye Res. 2022;89 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

