A novel anti-B7-H3 chimeric antigen receptor from a single-chain antibody library for immunotherapy of solid cancers
- PMID: 36159778
- PMCID: PMC9467911
- DOI: 10.1016/j.omto.2022.08.008
A novel anti-B7-H3 chimeric antigen receptor from a single-chain antibody library for immunotherapy of solid cancers
Abstract
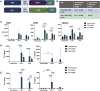
B7-H3 (CD276) has emerged as a target for cancer immunotherapy by virtue of consistent expression in many malignancies, relative absence from healthy tissues, and an emerging role as a driver of tumor immune inhibition. Recent studies have reported B7-H3 to be a suitable target for chimeric antigen receptor-modified T cell (CAR-T) therapy using CARs constructed from established anti-B7-H3 antibodies converted into single-chain Fv format (scFv). We constructed and screened binders in an scFv library to generate a new anti-B7-H3 CAR-T with favorable properties. This allowed access to numerous specificities ready formatted for CAR evaluation. Selected anti-human B7-H3 scFvs were readily cloned into CAR-T and evaluated for anti-tumor reactivity in cytotoxicity, cytokine, and proliferation assays. Two binders with divergent complementarity determining regions were found to show optimal antigen-specific cytotoxicity and cytokine secretion. One binder in second-generation CD28-CD3ζ CAR format induced sustained in vitro proliferation on repeat antigen challenge. The lead candidate CAR-T also demonstrated in vivo activity in a resistant neuroblastoma model. An empirical approach to B7-H3 CAR-T discovery through screening of novel scFv sequences in CAR-T format has led to the identification of a new construct with sustained proliferative capacity warranting further evaluation.
Keywords: B7-H3; CAR-T cell; neuroblastoma; pediatric cancer; phage display; solid tumor.
© 2022 The Author(s).
Conflict of interest statement
John Anderson declares founder shares in Autolus Ltd and collaborations with Roche and ALX-Oncology.
Figures






References
-
- Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N., et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. - PMC - PubMed
-
- Mohty M., Gautier J., Malard F., Aljurf M., Bazarbachi A., Chabannon C., Kharfan-Dabaja M.A., Savani B.N., Huang H., Kenderian S., et al. CD19 chimeric antigen receptor-T cells in B-cell leukemia and lymphoma: current status and perspectives. Leukemia. 2019;33:2767–2778. - PubMed
-
- Ramakrishna S., Barsan V., Mackall C. Prospects and challenges for use of CAR T cell therapies in solid tumors. Expert Opin. Biol. Ther. 2020;20:503–516. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

